正在加载图片...
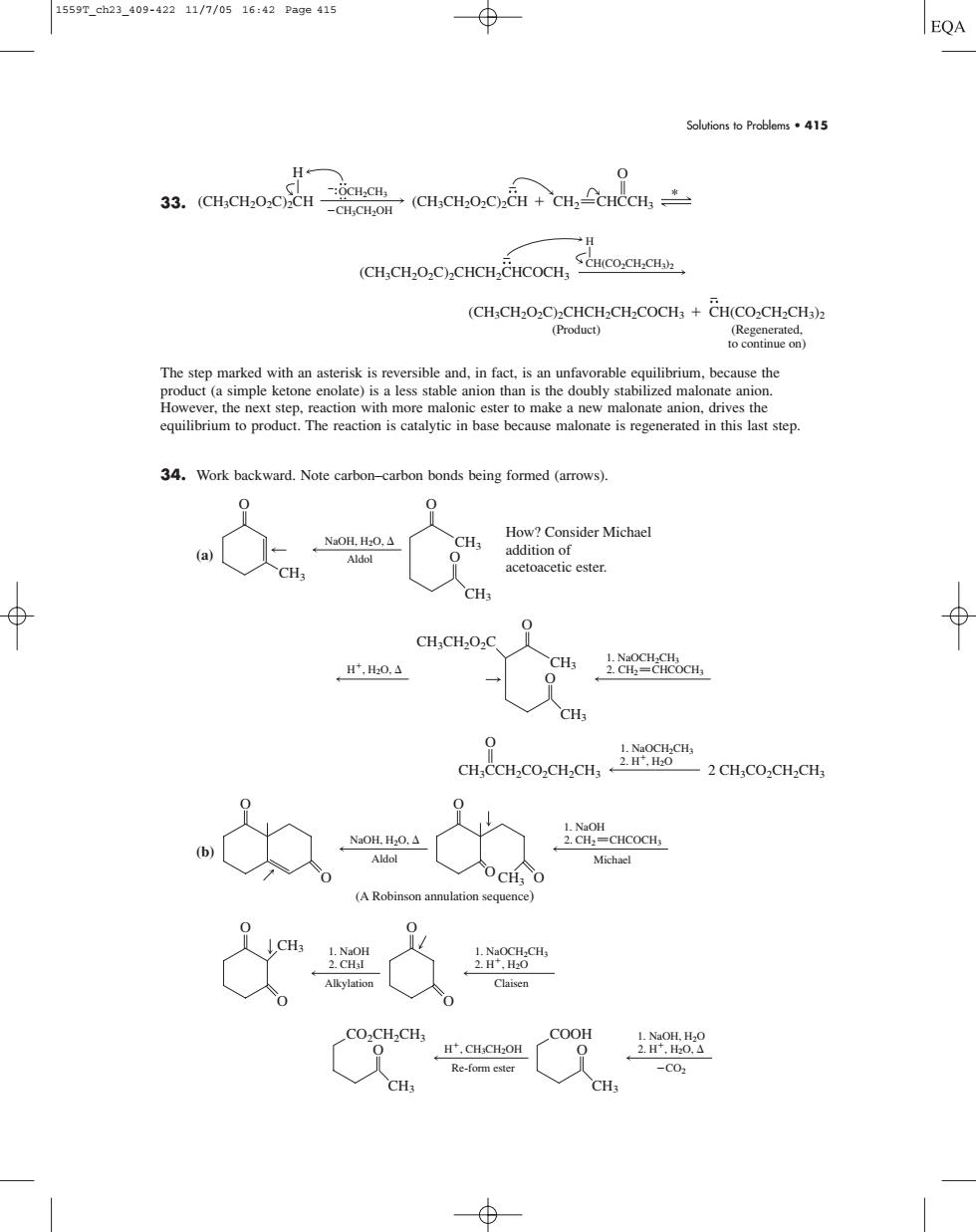
1559T_ch23_409-42211/7/0516:42Pa9e415 EQA Solutions to Problems.415 33.(CHCH2OzC)CH- -CH-CH-OH .(CH.CH.O.C.CH +CH.CHCCH (CH,CHOC)CHCH.CHCOCH,COCC CH.CHO..C..CHC 34.Work backward.Note carbon-carbon bonds being formed (arrows). acetoacetic ester. CH ⊕ CH,CH.O.C H,H0. CHs 89c -2 CH. NaOH.HO. CHCOCH. Michael Claisen 广.CH.CH.OH COOH 上08 Re-form ester -C0 CH: Solutions to Problems • 415 33. The step marked with an asterisk is reversible and, in fact, is an unfavorable equilibrium, because the product (a simple ketone enolate) is a less stable anion than is the doubly stabilized malonate anion. However, the next step, reaction with more malonic ester to make a new malonate anion, drives the equilibrium to product. The reaction is catalytic in base because malonate is regenerated in this last step. 34. Work backward. Note carbon–carbon bonds being formed (arrows). (a) (b) CH3 O CO2CH2CH3 CH3 O COOH Re-form ester H, CH3CH2OH 1. NaOH, H2O CO2 2. H, H2O, 1. NaOH Alkylation 2. CH3I 1. NaOCH2CH3 Claisen 2. H, H2O O O CH3 O O NaOH, H2O, Aldol 1. NaOH Michael 2. CH2 CHCOCH3 O O O O (A Robinson annulation sequence) O CH3 O 2. H, H2O 1. NaOCH2CH3 CH3CCH2CO2CH2CH3 2 CH3CO2CH2CH3 CH3 CH3 O O 2. CH2 CHCOCH3 1. NaOCH2CH3 H, H2O, CH3CH2O2C NaOH, H2O, Aldol CH3 CH3 CH3 O O O How? Consider Michael addition of acetoacetic ester. (CH3CH2O2C)2CHCH2CH2COCH3 CH(CO2CH2CH3)2 (Regenerated, to continue on) (Product) (CH3CH2O2C)2CHCH2CHCOCH3 CH(CO2CH2CH3)2 H CH3CH2OH OCH2CH3 (CH3CH2O2C)2CH H (CH3CH2O2C)2CH CH2 CHCCH3 O * 1559T_ch23_409-422 11/7/05 16:42 Page 415����������