正在加载图片...
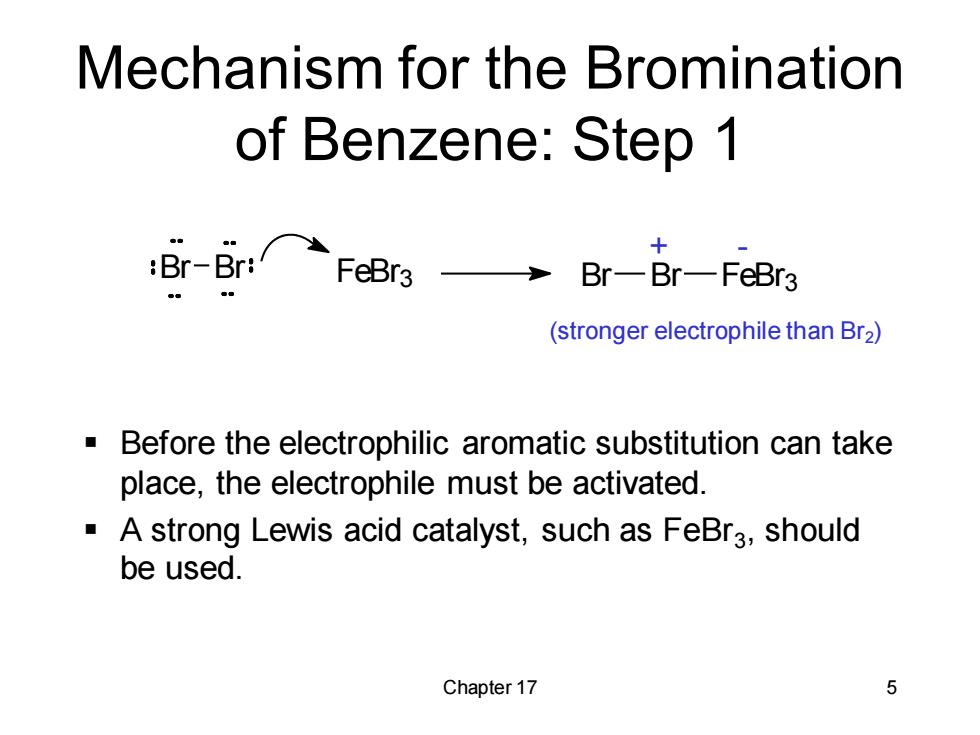
Mechanism for the Bromination of Benzene:Step 1 :Br--Br:FeBra3→ 十 Br-Br-FeBr3 (stronger electrophile than Br2) Before the electrophilic aromatic substitution can take place,the electrophile must be activated. A strong Lewis acid catalyst,such as FeBra,should be used. Chapter 17 5Chapter 17 5 Mechanism for the Bromination of Benzene: Step 1 ▪ Before the electrophilic aromatic substitution can take place, the electrophile must be activated. ▪ A strong Lewis acid catalyst, such as FeBr3 , should be used. Br Br FeBr3 Br Br FeBr3 + - (stronger electrophile than Br2)