正在加载图片...
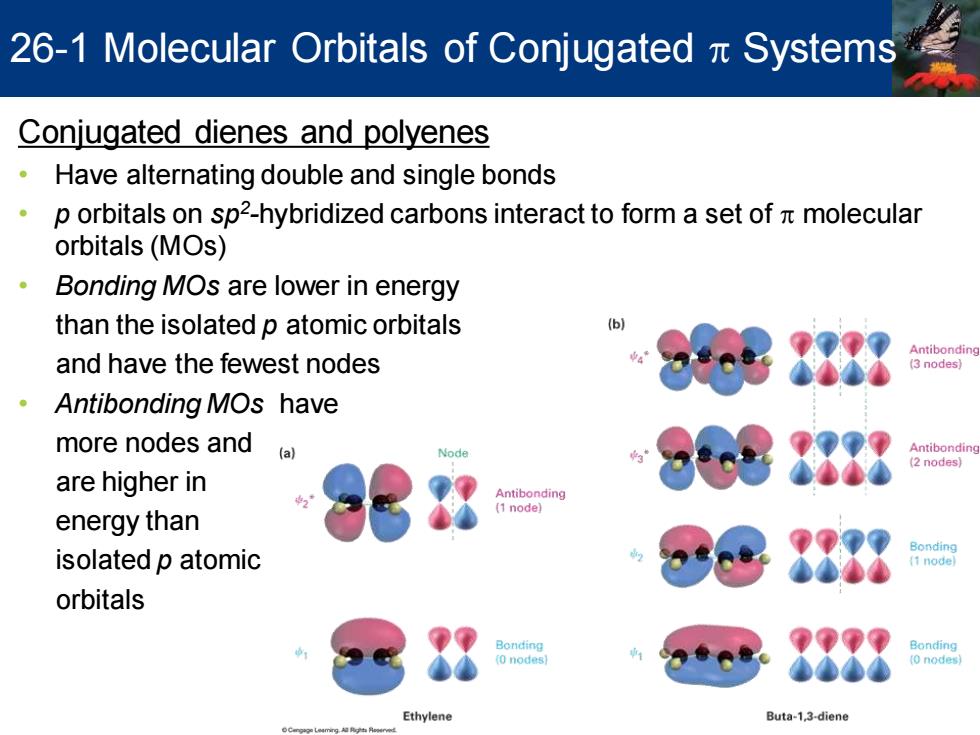
26-1 Molecular Orbitals of Conjugated Systems Conjugated dienes and polyenes Have alternating double and single bonds 。 p orbitals on sp2-hybridized carbons interact to form a set of molecular orbitals(MOs) 。 Bonding MOs are lower in energy than the isolated p atomic orbitals b Antibonding and have the fewest nodes 3 nodes ·Antibonding MOs have more nodes and (a) Antibonding 2 nodes) are higher in Antibonding (1 node) energy than isolated p atomic orbitals Bonding Bonding 0 nodes (0 nodes Ethylene Buta-1,3-diene Conjugated dienes and polyenes • Have alternating double and single bonds • p orbitals on sp2 -hybridized carbons interact to form a set of p molecular orbitals (MOs) • Bonding MOs are lower in energy than the isolated p atomic orbitals and have the fewest nodes • Antibonding MOs have more nodes and are higher in energy than isolated p atomic orbitals 26-1 Molecular Orbitals of Conjugated p Systems