正在加载图片...
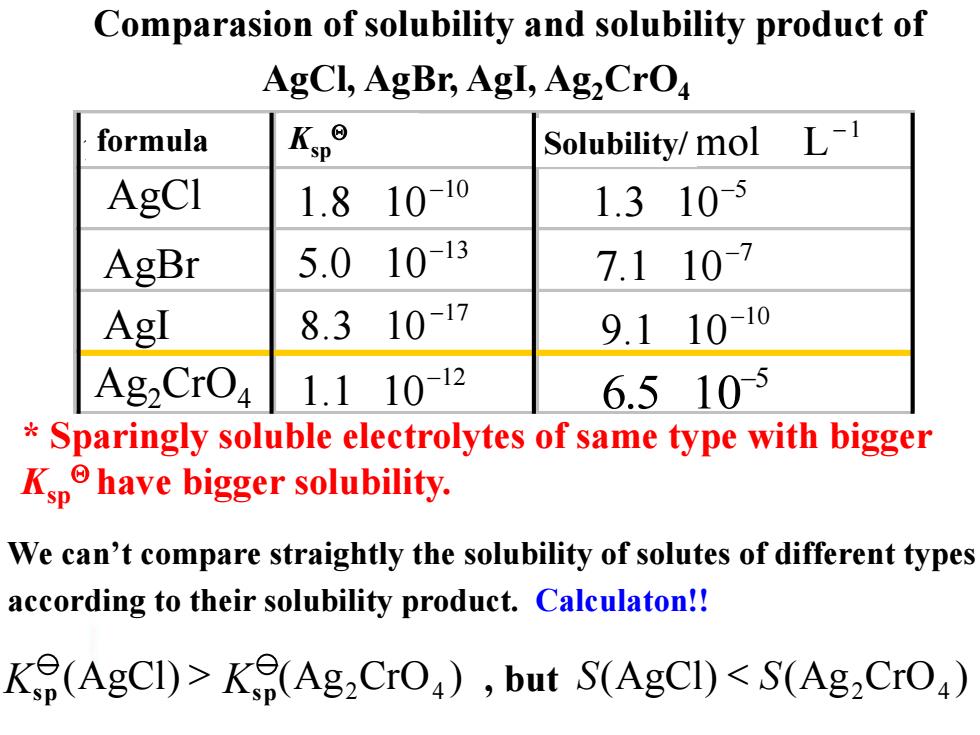
Comparasion of solubility and solubility product of AgCl,AgBr,AgI,Ag,CrO formula Kp° Solubility/mol L-1 AgCI 1.8 10-10 1.310-5 AgBr 5.0 10-13 7.110-7 AgI 8.310-17 9.11010 Ag2CrO4 1.11012 6.5105 Sparingly soluble electrolytes of same type with bigger Kphave bigger solubility. We can't compare straightly the solubility of solutes of different types according to their solubility product.Calculaton!! Ke(AgCI)>Ke(Ag2CrO),but S(AgCI)<S(Ag2CrO)(AgCl) (Ag CrO ) S S 2 4 < We can’t compare straightly the solubility of solutes of different types according to their solubility product. Calculaton!! * Sparingly soluble electrolytes of same type with bigger Ksp have bigger solubility. (AgCl) (Ag CrO ) 2 4 K > sp Ksp Comparasion of solubility and solubility product of AgCl, AgBr, AgI, Ag2CrO4 , but 分子式 溶度积 溶解度/ AgBr AgI AgCl 5 6.5 10- 1 mol L - 10 1.8 10- 13 5.0 10- 17 8.3 10 - 12 1.1 10- 10 9.1 10- 7 7.1 10- 5 1.3 10- Ag2CrO4 formula Ksp Solubility/