正在加载图片...
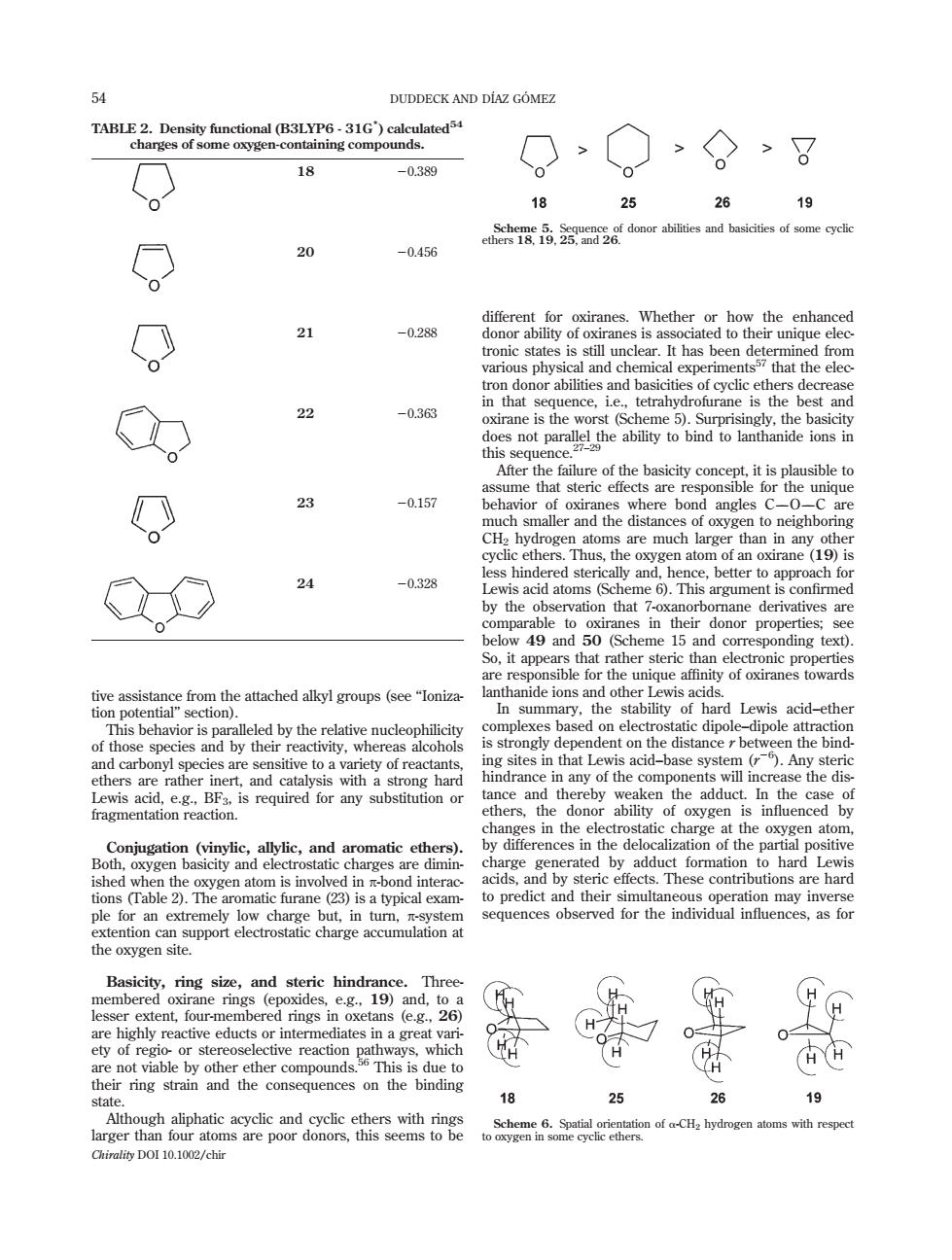
DUDDECK AND DIAZ GOMEZ > >。>8 18 -0389 18 25 26 19 -0.456 co end aeof meye 20 -0.28 22 -0363 oxirane is the worst (Scheme5).Surprisingly,the basicity he ability to bind to lanthanide ions -0.157 之上物 CH -0329 Q 24 ponding text) unique affinity of oxiranes towards the o theth ity,wher ing s in that Le ers are rath acid-base syste the adduc ramentation reaction. any s subsiong hard the case xygen b Conjugation (vinylic,allylic,and aromatic ethe ion of the ie inu effects.These contributions are hard to predic extent fourm rings in oxctans g26) 25 26 19 Chirality DOI 10.1002/chir tive assistance from the attached alkyl groups (see ‘‘Ionization potential’’ section). This behavior is paralleled by the relative nucleophilicity of those species and by their reactivity, whereas alcohols and carbonyl species are sensitive to a variety of reactants, ethers are rather inert, and catalysis with a strong hard Lewis acid, e.g., BF3, is required for any substitution or fragmentation reaction. Conjugation (vinylic, allylic, and aromatic ethers). Both, oxygen basicity and electrostatic charges are diminished when the oxygen atom is involved in p-bond interactions (Table 2). The aromatic furane (23) is a typical example for an extremely low charge but, in turn, p-system extention can support electrostatic charge accumulation at the oxygen site. Basicity, ring size, and steric hindrance. Threemembered oxirane rings (epoxides, e.g., 19) and, to a lesser extent, four-membered rings in oxetans (e.g., 26) are highly reactive educts or intermediates in a great variety of regio- or stereoselective reaction pathways, which are not viable by other ether compounds.56 This is due to their ring strain and the consequences on the binding state. Although aliphatic acyclic and cyclic ethers with rings larger than four atoms are poor donors, this seems to be different for oxiranes. Whether or how the enhanced donor ability of oxiranes is associated to their unique electronic states is still unclear. It has been determined from various physical and chemical experiments57 that the electron donor abilities and basicities of cyclic ethers decrease in that sequence, i.e., tetrahydrofurane is the best and oxirane is the worst (Scheme 5). Surprisingly, the basicity does not parallel the ability to bind to lanthanide ions in this sequence.27–29 After the failure of the basicity concept, it is plausible to assume that steric effects are responsible for the unique behavior of oxiranes where bond angles COC are much smaller and the distances of oxygen to neighboring CH2 hydrogen atoms are much larger than in any other cyclic ethers. Thus, the oxygen atom of an oxirane (19) is less hindered sterically and, hence, better to approach for Lewis acid atoms (Scheme 6). This argument is confirmed by the observation that 7-oxanorbornane derivatives are comparable to oxiranes in their donor properties; see below 49 and 50 (Scheme 15 and corresponding text). So, it appears that rather steric than electronic properties are responsible for the unique affinity of oxiranes towards lanthanide ions and other Lewis acids. In summary, the stability of hard Lewis acid–ether complexes based on electrostatic dipole–dipole attraction is strongly dependent on the distance r between the binding sites in that Lewis acid–base system (r 26 ). Any steric hindrance in any of the components will increase the distance and thereby weaken the adduct. In the case of ethers, the donor ability of oxygen is influenced by changes in the electrostatic charge at the oxygen atom, by differences in the delocalization of the partial positive charge generated by adduct formation to hard Lewis acids, and by steric effects. These contributions are hard to predict and their simultaneous operation may inverse sequences observed for the individual influences, as for TABLE 2. Density functional (B3LYP6 - 31G* ) calculated54 charges of some oxygen-containing compounds. 18 20.389 20 20.456 21 20.288 22 20.363 23 20.157 24 20.328 Scheme 5. Sequence of donor abilities and basicities of some cyclic ethers 18, 19, 25, and 26. Scheme 6. Spatial orientation of a-CH2 hydrogen atoms with respect to oxygen in some cyclic ethers. 54 DUDDECK AND DI´AZ GO´ MEZ Chirality DOI 10.1002/chir