正在加载图片...
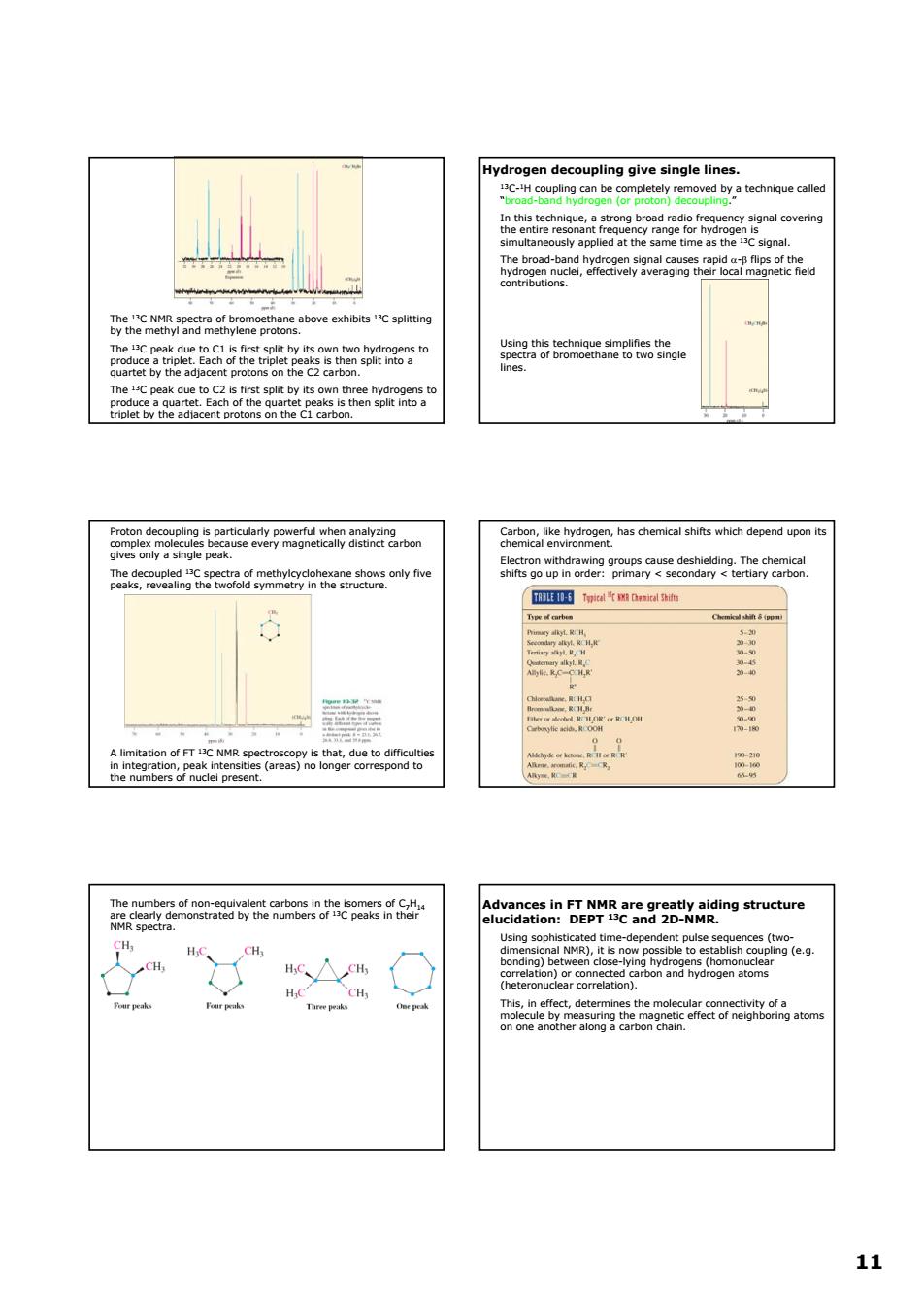
Hydrogen decoupling give single lines. 9n at oythfom 西tmaa 用 AtcsiBDEpR2ngr2aangstuctwre △ SNge9 % 11 11 The 13C NMR spectra of bromoethane above exhibits 13C splitting by the methyl and methylene protons. The 13C peak due to C1 is first split by its own two hydrogens to produce a triplet. Each of the triplet peaks is then split into a quartet by the adjacent protons on the C2 carbon. The 13C peak due to C2 is first split by its own three hydrogens to produce a quartet. Each of the quartet peaks is then split into a triplet by the adjacent protons on the C1 carbon. Hydrogen decoupling give single lines. 13C-1H coupling can be completely removed by a technique called “broad-band hydrogen (or proton) decoupling.” In this technique, a strong broad radio frequency signal covering the entire resonant frequency range for hydrogen is simultaneously applied at the same time as the 13C signal. The broad-band hydrogen signal causes rapid α-β flips of the hydrogen nuclei, effectively averaging their local magnetic field contributions. Using this technique simplifies the spectra of bromoethane to two single lines. Proton decoupling is particularly powerful when analyzing complex molecules because every magnetically distinct carbon gives only a single peak. The decoupled 13C spectra of methylcyclohexane shows only five peaks, revealing the twofold symmetry in the structure. A limitation of FT 13C NMR spectroscopy is that, due to difficulties in integration, peak intensities (areas) no longer correspond to the numbers of nuclei present. Carbon, like hydrogen, has chemical shifts which depend upon its chemical environment. Electron withdrawing groups cause deshielding. The chemical shifts go up in order: primary < secondary < tertiary carbon. The numbers of non-equivalent carbons in the isomers of C7H14 are clearly demonstrated by the numbers of 13C peaks in their NMR spectra. Advances in FT NMR are greatly aiding structure elucidation: DEPT 13C and 2D-NMR. Using sophisticated time-dependent pulse sequences (twodimensional NMR), it is now possible to establish coupling (e.g. bonding) between close-lying hydrogens (homonuclear correlation) or connected carbon and hydrogen atoms (heteronuclear correlation). This, in effect, determines the molecular connectivity of a molecule by measuring the magnetic effect of neighboring atoms on one another along a carbon chain