正在加载图片...
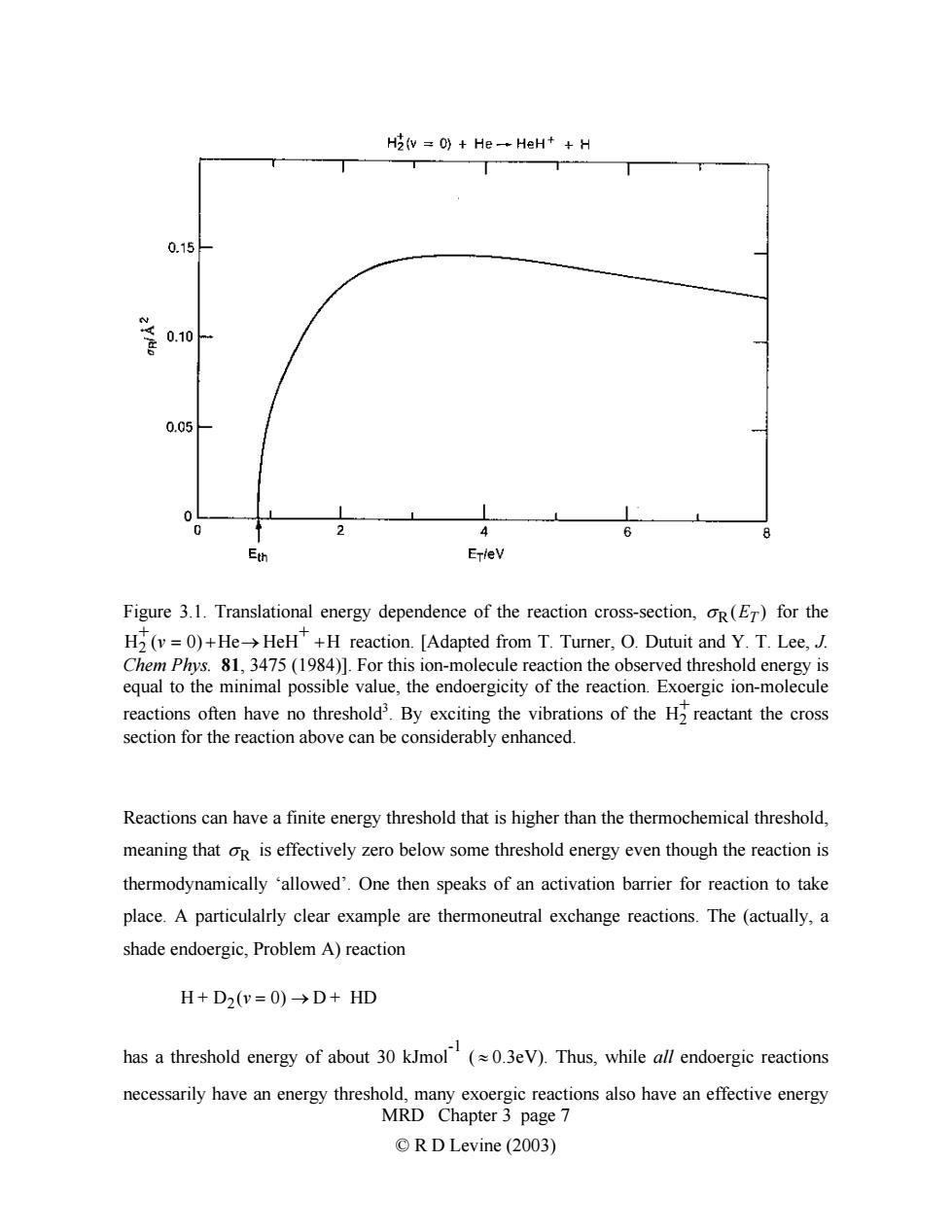
H2(v =0)He-HeH++H 0.15 0.10 0.05 0 4 6 8 E州 ErleV Figure 3.1.Translational energy dependence of the reaction cross-section,oR(ET)for the H(v=0)+He>HeH+H reaction.[Adapted from T.Turner,O.Dutuit and Y.T.Lee,J. Chem Phys.81,3475(1984)].For this ion-molecule reaction the observed threshold energy is equal to the minimal possible value,the endoergicity of the reaction.Exoergic ion-molecule reactions often have no threshold3.By exciting the vibrations of the H2 reactant the cross section for the reaction above can be considerably enhanced. Reactions can have a finite energy threshold that is higher than the thermochemical threshold, meaning that oR is effectively zero below some threshold energy even though the reaction is thermodynamically 'allowed'.One then speaks of an activation barrier for reaction to take place.A particulalrly clear example are thermoneutral exchange reactions.The (actually,a shade endoergic,Problem A)reaction H+D2(v=O)→D+HD has a threshold energy of about 30 kJmol(0.3eV).Thus,while all endoergic reactions necessarily have an energy threshold,many exoergic reactions also have an effective energy MRD Chapter 3 page 7 ©R D Levine(2003)Figure 3.1. Translational energy dependence of the reaction cross-section, σ R(ET ) for the reaction. [Adapted from T. Turner, O. Dutuit and Y. T. Lee, J. Chem Phys. 81, 3475 (1984)]. For this ion-molecule reaction the observed threshold energy is equal to the minimal possible value, the endoergicity of the reaction. Exoergic ion-molecule reactions often have no threshold H2 +(v = 0) +He→ HeH+ +H 3 . By exciting the vibrations of the H reactant the cross section for the reaction above can be considerably enhanced. 2 + Reactions can have a finite energy threshold that is higher than the thermochemical threshold, meaning that σ R is effectively zero below some threshold energy even though the reaction is thermodynamically ‘allowed’. One then speaks of an activation barrier for reaction to take place. A particulalrly clear example are thermoneutral exchange reactions. The (actually, a shade endoergic, Problem A) reaction H + D2(v = 0) → D + HD MRD Chapter 3 page 7 © R D Levine (2003) has a threshold energy of about 30 kJmol -1 ( ≈ 0.3eV). Thus, while all endoergic reactions necessarily have an energy threshold, many exoergic reactions also have an effective energy