正在加载图片...
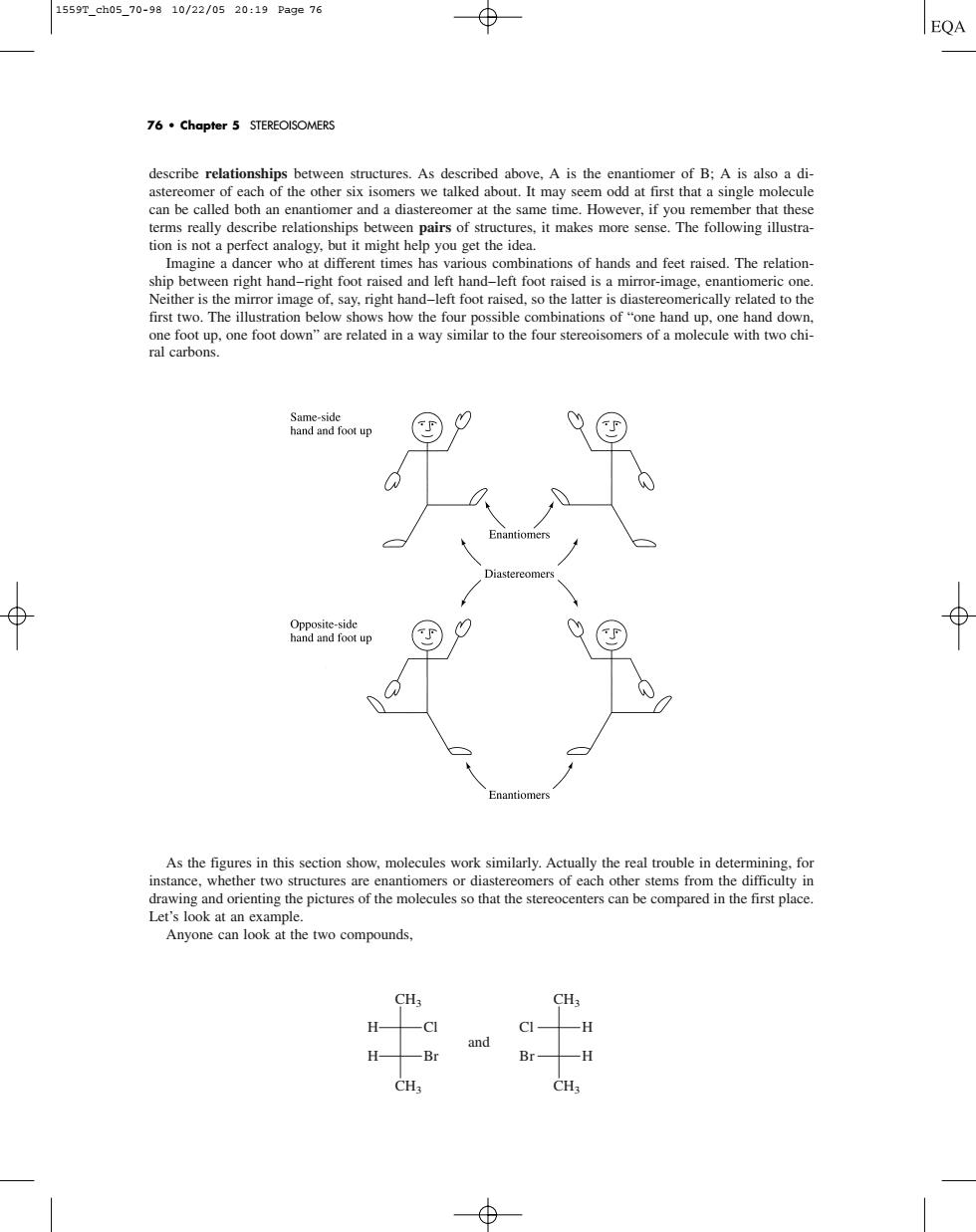
1559r_ch05.70-9810/22/0520:19Page76 76.Chapter 5 STEREOISOMERS describe relation reAs described above.is the antio mer of B:A is also a di can be called both n enantiomer and dastereomer at the same time.However.if you remember that these terms really describe relationships between pairs of structures,it makes more sense.The following illustra- p you get the i nations of hands and feet raised.The relation ship betwe en right hand-right foot raised and left hand-left foot raised is a mirror-image.enantiomeric on ither is The mage of say.right hand- -lelt foot raised,so the latter is ral carbons drawing and orienting the pictures of the molecules so that the stereocenters can be compared in the first place Let's look at an ample two compounds. CH; CH. H -C CI- -H and Br -H CHdescribe relationships between structures. As described above, A is the enantiomer of B; A is also a diastereomer of each of the other six isomers we talked about. It may seem odd at first that a single molecule can be called both an enantiomer and a diastereomer at the same time. However, if you remember that these terms really describe relationships between pairs of structures, it makes more sense. The following illustration is not a perfect analogy, but it might help you get the idea. Imagine a dancer who at different times has various combinations of hands and feet raised. The relationship between right hand–right foot raised and left hand–left foot raised is a mirror-image, enantiomeric one. Neither is the mirror image of, say, right hand–left foot raised, so the latter is diastereomerically related to the first two. The illustration below shows how the four possible combinations of “one hand up, one hand down, one foot up, one foot down” are related in a way similar to the four stereoisomers of a molecule with two chiral carbons. As the figures in this section show, molecules work similarly. Actually the real trouble in determining, for instance, whether two structures are enantiomers or diastereomers of each other stems from the difficulty in drawing and orienting the pictures of the molecules so that the stereocenters can be compared in the first place. Let’s look at an example. Anyone can look at the two compounds, CH3 CH3 H Cl Br and H CH3 CH3 H H Cl Br 76 • Chapter 5 STEREOISOMERS 1559T_ch05_70-98 10/22/05 20:19 Page 76