正在加载图片...
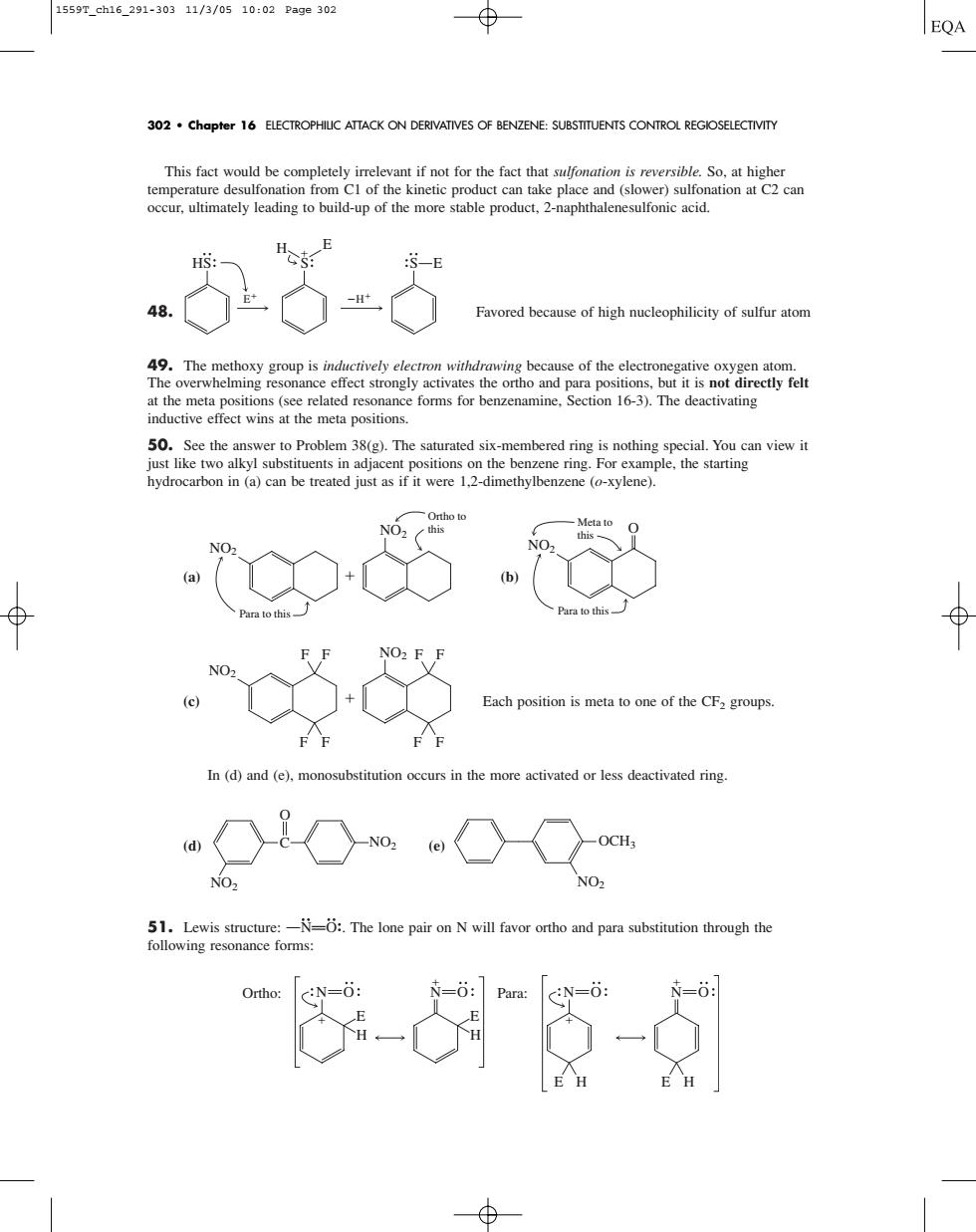
1559T_ch16_291-30311/3/0510:02Page302 ⊕ EQA 302.Chapter 16 ELECTROPHIUC ATTACK ON DERIVATIVES OF BENZENE:SUBSTITUENTS CONTROL REGIOSELECTIVITY occur.ultimately leading to build-up of the more stable product,2-naphthalenesulfonic aci -5 at the meta positions (sce related forms for benzenamins.Section 13).The deactivating inductive effect wins at the meta positions. 50.See the answer to Problem 38(g).The pecial.You can view it just like two alkyl substitucnts in adjacent positions on the benzene ring.For example.the starting nydrocarbon in (a)can be treated just as if it were 1,2-dimethylbenzene (o-xylene) Each position is meta to one of the CF2 groups. In(d)and (e).monosubstitution occurs in the more activated or less deactivated ring wOw.OCam :The lone pair on N will favor ortho and hrough 心-“5-女 This fact would be completely irrelevant if not for the fact that sulfonation is reversible. So, at higher temperature desulfonation from C1 of the kinetic product can take place and (slower) sulfonation at C2 can occur, ultimately leading to build-up of the more stable product, 2-naphthalenesulfonic acid. 48. Favored because of high nucleophilicity of sulfur atom 49. The methoxy group is inductively electron withdrawing because of the electronegative oxygen atom. The overwhelming resonance effect strongly activates the ortho and para positions, but it is not directly felt at the meta positions (see related resonance forms for benzenamine, Section 16-3). The deactivating inductive effect wins at the meta positions. 50. See the answer to Problem 38(g). The saturated six-membered ring is nothing special. You can view it just like two alkyl substituents in adjacent positions on the benzene ring. For example, the starting hydrocarbon in (a) can be treated just as if it were 1,2-dimethylbenzene (o-xylene). (a) (b) (c) Each position is meta to one of the CF2 groups. In (d) and (e), monosubstitution occurs in the more activated or less deactivated ring. (d) (e) 51. Lewis structure: OONPOOS. The lone pair on N will favor ortho and para substitution through the following resonance forms: E N H O E Ortho: N Para: H O E N H E H O N O NO2 OCH3 NO2 C NO2 O NO2 F F F F NO2 F F F F NO2 Para to this Meta to this O NO2 Para to this NO2 Ortho to this H E S H E HS S E 302 • Chapter 16 ELECTROPHILIC ATTACK ON DERIVATIVES OF BENZENE: SUBSTITUENTS CONTROL REGIOSELECTIVITY 1559T_ch16_291-303 11/3/05 10:02 Page 302���������