正在加载图片...
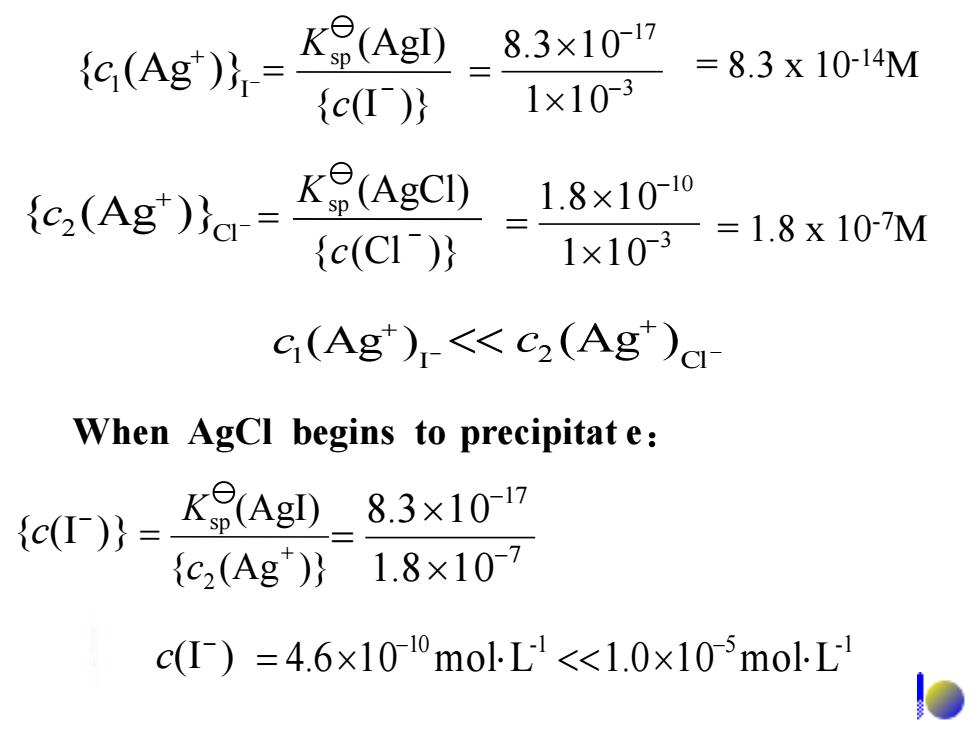
8.3×1017 {C(Ag)}= K(AgI) =8.3x10-14M {c(I)} 1×103 (e(Ag') K(AgCI) 1.8×1010 c(CI) 1×103 =1.8x107M G(Ag)-<<c2(Ag)a When AgCl begins to precipitat e: c()=K(Agl)_8.3x107 {c2(Ag)}1.8×107 c(I)=4.6×100molL<1.0x105molL - + 1 I c (Ag ) - + 2 Cl << c (Ag ) 10 -1 5 -1 = 4.61 0 molL <<1.01 0 molL - - (I ) - c - + 1 I {c (Ag )} 3 17 1 1 0 8.3 1 0 - - = { (I )} (AgI) sp - = c K - + 2 Cl {c (Ag )} 3 10 1 1 0 1.8 1 0 - - = { (Cl )} (AgCl) sp - = c K { (I )} - c { (Ag )} (AgI) 2 sp + = c K 7 17 1.8 10 8.3 10 - - = = 8.3 x 10-14M = 1.8 x 10-7M When AgCl begins to precipitat e: