正在加载图片...
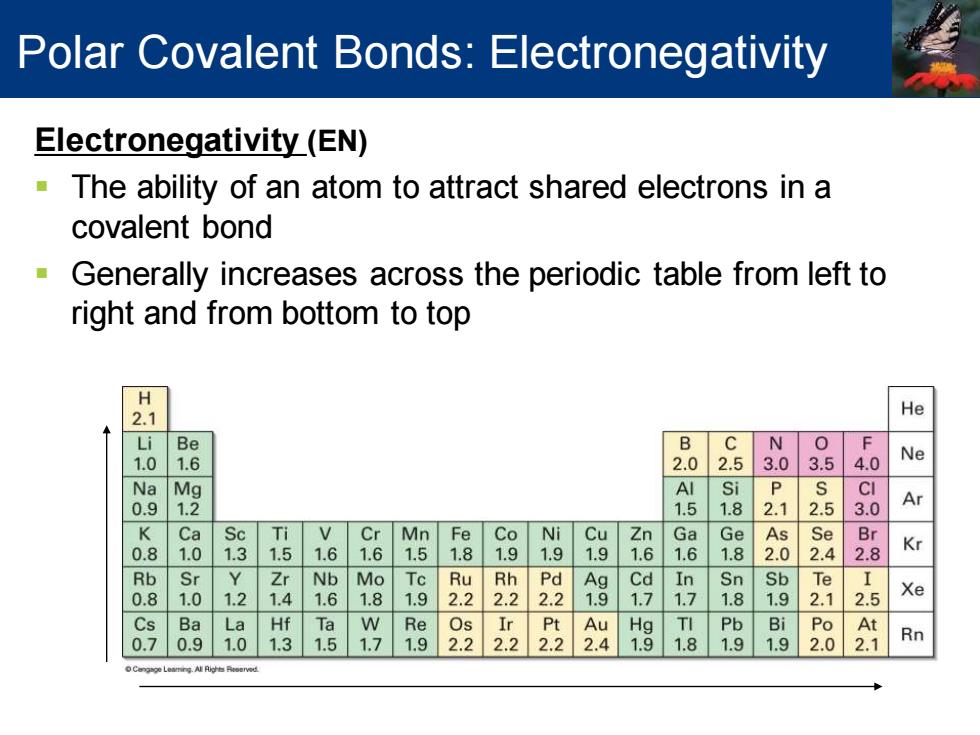
Polar Covalent Bonds:Electronegativity Electronegativity(EN) The ability of an atom to attract shared electrons in a covalent bond Generally increases across the periodic table from left to right and from bottom to top 2.1 He i Be B C N 1. 6 2.0 2.5 3.0 3.5 4.0 Ne N Mg Al i P CI 0. 2 1.5 1. 2.5 Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br . 0 1.3 1.5 1.6 1.6 5 1.8 9 1.9 9 6 1.6 1.8 2.0 2.4 2.8 Kr Rb Sr Zr Nb Mo Ru 22 22 铝 Cd In Sn Sb Te 8 1 1.2 4 6 8 1.9 2 7 7 8 9 2.1 2.5 Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At 0.7 .9 1.0 1.3 1.5 1.7 1.9 2.2 2.2 2.22.4 1.9 1.81.9 1.9 2.0 2.1 RnElectronegativity (EN) ▪ The ability of an atom to attract shared electrons in a covalent bond ▪ Generally increases across the periodic table from left to right and from bottom to top Polar Covalent Bonds: Electronegativity