正在加载图片...
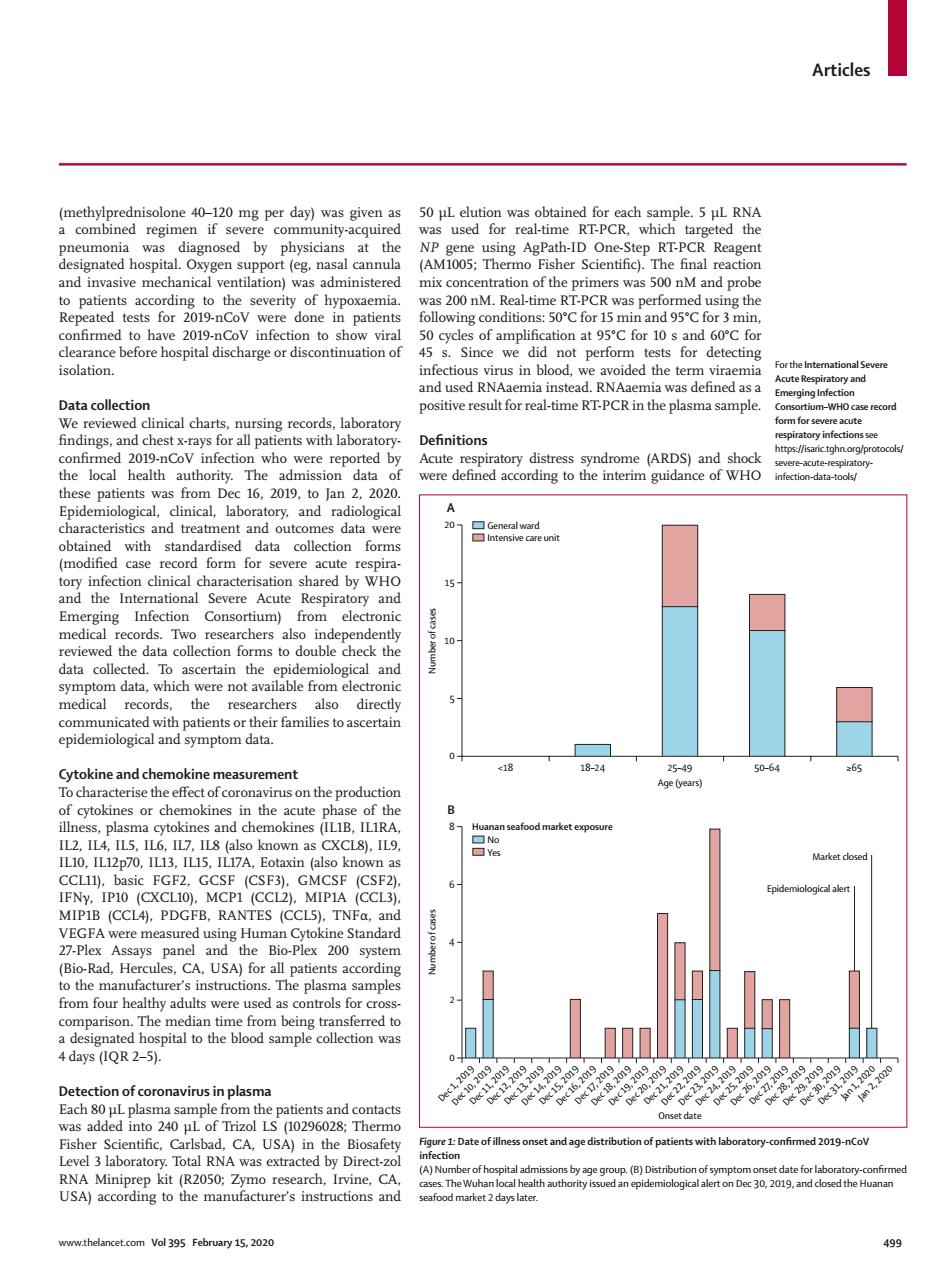
Articles at the and invasive mechanical ventilation)was administered mix con centration of the primers was 500 nM and probe findings,an i-ncyn ne (ARDS)and shoc The gtoodata-ods 2019 nd nd treat (modified case form for severe acute 15 Emerging Infection Consortium) from elec records. the chers also directh piaemoiCcdtspar也heartamisoasceain 18-34 8 known as CXCL8).IL9 GMCSE (CCI 200 system manu instructio The plasma rred to LS (0296028:Therm s with EV-5O0 rmed 2019-nCov Total RNA as extracted by Directo al admi The Vol395 499Articles www.thelancet.com Vol 395 February 15, 2020 499 (methylprednisolone 40–120 mg per day) was given as a combined regimen if severe community-acquired pneumonia was diagnosed by physicians at the designated hospital. Oxygen support (eg, nasal cannula and invasive mechanical ventilation) was administered to patients according to the severity of hypoxaemia. Repeated tests for 2019-nCoV were done in patients confirmed to have 2019-nCoV infection to show viral clearance before hospital discharge or discontinuation of isolation. Data collection We reviewed clinical charts, nursing records, laboratory findings, and chest x-rays for all patients with laboratoryconfirmed 2019-nCoV infection who were reported by the local health authority. The admission data of these patients was from Dec 16, 2019, to Jan 2, 2020. Epidemiological, clinical, laboratory, and radiological characteristics and treatment and outcomes data were obtained with standardised data collection forms (modified case record form for severe acute respiratory infection clinical characterisation shared by WHO and the International Severe Acute Respiratory and Emerging Infection Consortium) from electronic medical records. Two researchers also independently reviewed the data collection forms to double check the data collected. To ascertain the epidemiological and symptom data, which were not available from electronic medical records, the researchers also directly communicated with patients or their families to ascertain epidemiological and symptom data. Cytokine and chemokine measurement To characterise the effect of coronavirus on the production of cytokines or chemokines in the acute phase of the illness, plasma cytokines and chemokines (IL1B, IL1RA, IL2, IL4, IL5, IL6, IL7, IL8 (also known as CXCL8), IL9, IL10, IL12p70, IL13, IL15, IL17A, Eotaxin (also known as CCL11), basic FGF2, GCSF (CSF3), GMCSF (CSF2), IFNγ, IP10 (CXCL10), MCP1 (CCL2), MIP1A (CCL3), MIP1B (CCL4), PDGFB, RANTES (CCL5), TNFα, and VEGFA were measured using Human Cytokine Standard 27-Plex Assays panel and the Bio-Plex 200 system (Bio-Rad, Hercules, CA, USA) for all patients according to the manufacturer’s instructions. The plasma samples from four healthy adults were used as controls for crosscomparison. The median time from being transferred to a designated hospital to the blood sample collection was 4 days (IQR 2–5). Detection of coronavirus in plasma Each 80 µL plasma sample from the patients and contacts was added into 240 µL of Trizol LS (10296028; Thermo Fisher Scientific, Carlsbad, CA, USA) in the Biosafety Level 3 laboratory. Total RNA was extracted by Direct-zol RNA Miniprep kit (R2050; Zymo research, Irvine, CA, USA) according to the manufacturer’s instructions and 50 µL elution was obtained for each sample. 5 µL RNA was used for real-time RT-PCR, which targeted the NP gene using AgPath-ID One-Step RT-PCR Reagent (AM1005; Thermo Fisher Scientific). The final reaction mix concentration of the primers was 500 nM and probe was 200 nM. Real-time RT-PCR was performed using the following conditions: 50°C for 15 min and 95°C for 3 min, 50 cycles of amplification at 95°C for 10 s and 60°C for 45 s. Since we did not perform tests for detecting infectious virus in blood, we avoided the term viraemia and used RNAaemia instead. RNAaemia was defined as a positive result for real-time RT-PCR in the plasma sample. Definitions Acute respiratory distress syndrome (ARDS) and shock were defined according to the interim guidance of WHO For the International Severe Acute Respiratory and Emerging Infection Consortium–WHO case record form for severe acute respiratory infections see https://isaric.tghn.org/protocols/ severe-acute-respiratoryinfection-data-tools/ Figure 1: Date of illness onset and age distribution of patients with laboratory-confirmed 2019-nCoV infection (A) Number of hospital admissions by age group. (B) Distribution of symptom onset date for laboratory-confirmed cases. The Wuhan local health authority issued an epidemiological alert on Dec 30, 2019, and closed the Huanan seafood market 2 days later. 0 <18 18–24 25–49 50–64 ≥65 5 10 15 20 Number of cases Age (years) A General ward Intensive care unit Dec 1, 2019 Dec 10, 2019 Dec 11, 2019 Dec 12, 2019 Dec 13, 2019 Dec 14, 2019 Dec 15, 2019 Dec 16, 2019 Dec 17, 2019 Dec 18, 2019 Dec 19, 2019 Dec 20, 2019 Dec 21, 2019 Dec 22, 2019 Dec 23, 2019 Dec 24, 2019 Dec 25, 2019 Dec 26, 2019 Dec 27, 2019 Dec 28, 2019 Dec 29, 2019 Dec 30, 2019 Dec 31, 2019 Jan 1, 2020 Jan 2, 2020 0 2 4 6 8 Number of cases Onset date B No Yes Huanan seafood market exposure Epidemiological alert Market closed