正在加载图片...
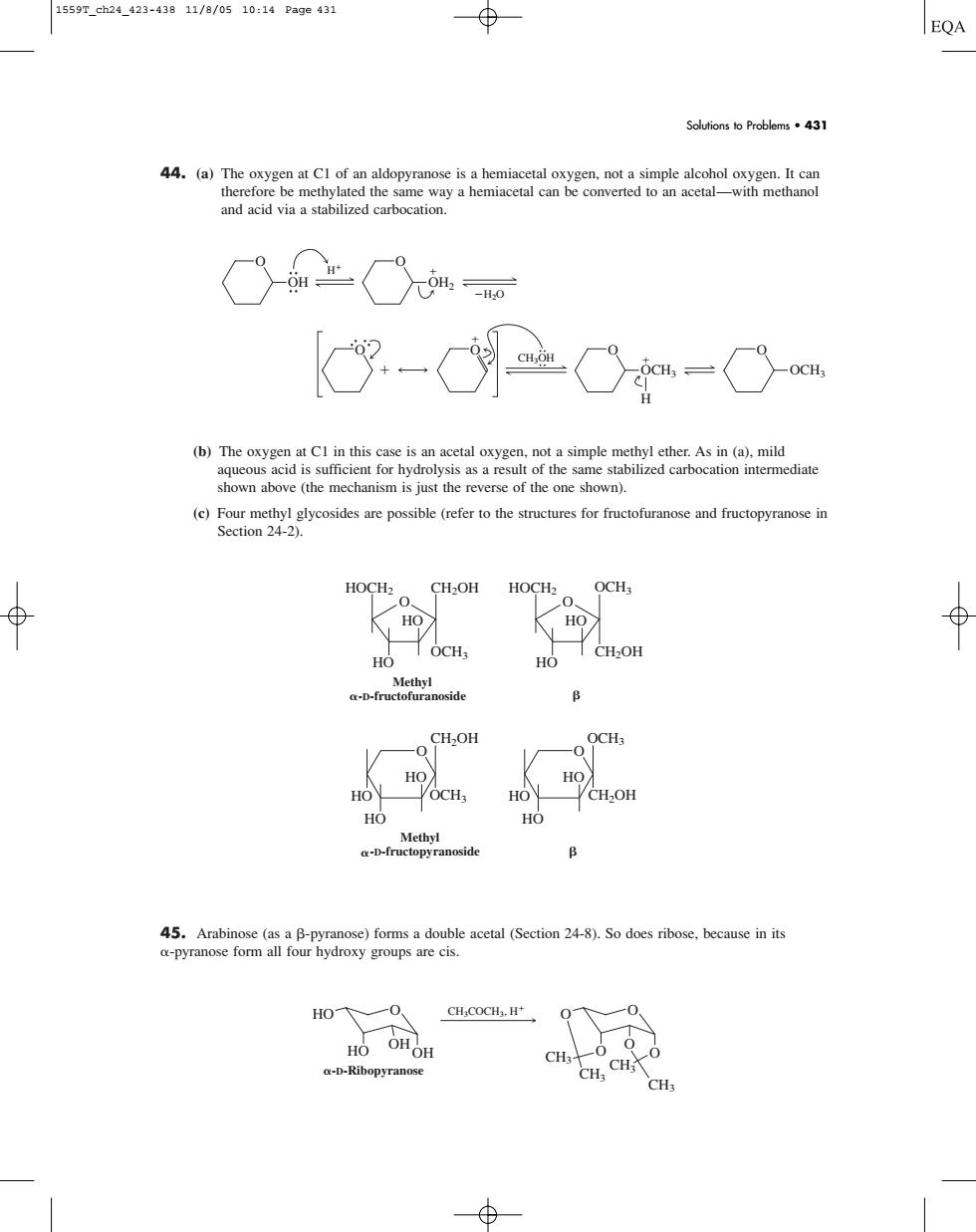
1559T_ch24_423-43811/8/0510:14Page431 ⊕ EQA Solutions to Problems.431 44.(a)The and acid via a stabilized carbocation. 土Cm 8--Cm (b)The oxygen at Cl in this case is an acetal oxygen,not a simple methyl ether.As in (a).mild (e)Four methyl glycosides are possible (refer to the structures for fructofuranose and fructopyranose in Section 24-2) HOCH2 CH2OH HOCH2 OCH3 ⊕ KI HO HO HO 1 OCH, HO B CH.OH HO HO VOCH HO CH,OH H HO ann 45.Arabinose(as a B-pyranose)forms a double acetal (Section 24-8).So does ribose.because in its a-pyranose form all four hydroxy groups are cis. CH;COCH.H HO OHO CH; -D-Ribopyrano H.CH 44. (a) The oxygen at C1 of an aldopyranose is a hemiacetal oxygen, not a simple alcohol oxygen. It can therefore be methylated the same way a hemiacetal can be converted to an acetal—with methanol and acid via a stabilized carbocation. (b) The oxygen at C1 in this case is an acetal oxygen, not a simple methyl ether. As in (a), mild aqueous acid is sufficient for hydrolysis as a result of the same stabilized carbocation intermediate shown above (the mechanism is just the reverse of the one shown). (c) Four methyl glycosides are possible (refer to the structures for fructofuranose and fructopyranose in Section 24-2). 45. Arabinose (as a -pyranose) forms a double acetal (Section 24-8). So does ribose, because in its -pyranose form all four hydroxy groups are cis. HO O HO OHOH CH3 CH3 CH3 O O CH3 O O O CH3COCH3, H -D-Ribopyranose HO HO HO O CH2OH OCH3 HO HO HO O CH2OH OCH3 -D-fructopyranoside Methyl O HO -D-fructofuranoside Methyl HO OCH3 CH2OH O HO HO HOCH2 HOCH2 OCH3 CH2OH O O CH3OH O OCH3 O OCH3 H H O OH H2O O OH2 Solutions to Problems • 431 1559T_ch24_423-438 11/8/05 10:14 Page 431����