正在加载图片...
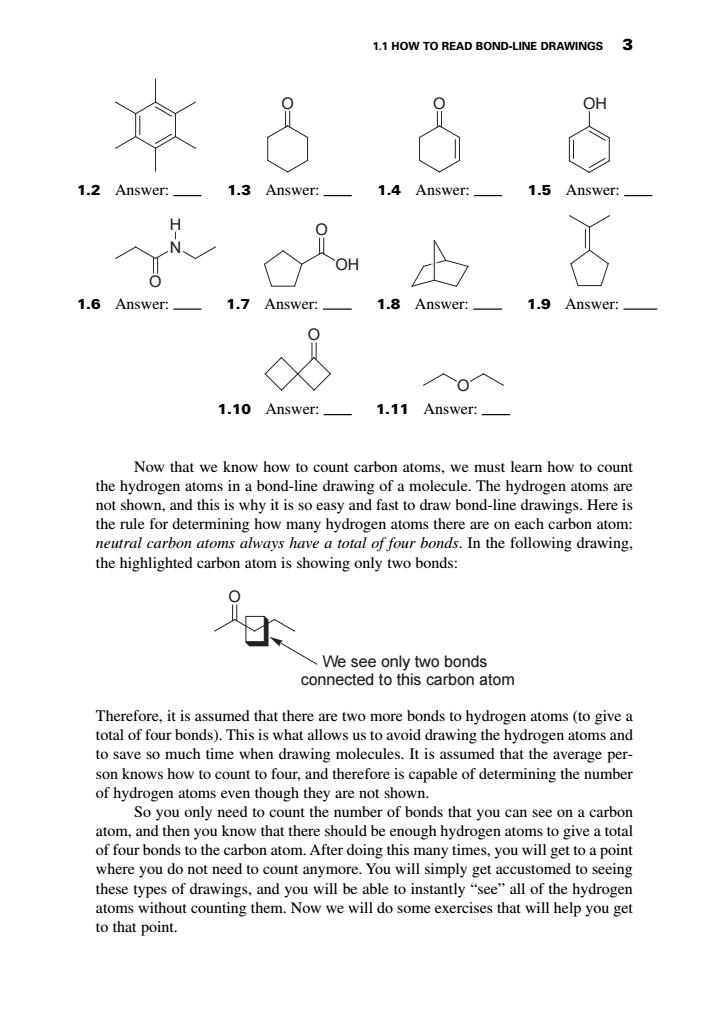
1.1 HOW TO READ BOND-LINE DRAWINGS 3 女3&8 Answer:_ 3 Answer: 1.4 Answer:_ Answer 1.6 Answer:_ 1.7 Answer:_ 1.8 Answer:_ 1.9 Answer: 入0入 1.10 Answer:_1.11 Answer:_ Now that we know how to count carbon atoms,we must learn how to count the hydrogen atoms in a bond-line drawing of a molecule.The hydrogen atoms are not shown,and this is why it is so easy and fast to draw bond-line drawings.Here is neutral carbon atoms always ha We see only two bonds connected to this carbon atom Therefore,it is assumed that there are two more bonds to hydrogen atoms(to give a total of four bonds).This is what allows us to avoid drawing the hydrogen atoms and to save so much time when drawing molecules.It is assumed that the average per- son knows therefore is capable of the umber of hydrogen atoms even though they are not shown. So you only need to count the number of bonds that you can see on a carbon atom,and then you know that there should be enough hydrogen atoms to give a total of four bonds to the carbon atom.After doing this many times,you will get to a point where you do not need to count anymore.You will simply get accustomed to seeing these types of drawings.and you will be able to instantly"ee all of the hydrogen atoms without counting them.Now we will do some exercises that will help you get to that point. Now that we know how to count carbon atoms, we must learn how to count the hydrogen atoms in a bond-line drawing of a molecule. The hydrogen atoms are not shown, and this is why it is so easy and fast to draw bond-line drawings. Here is the rule for determining how many hydrogen atoms there are on each carbon atom: neutral carbon atoms always have a total of four bonds. In the following drawing, the highlighted carbon atom is showing only two bonds: Therefore, it is assumed that there are two more bonds to hydrogen atoms (to give a total of four bonds). This is what allows us to avoid drawing the hydrogen atoms and to save so much time when drawing molecules. It is assumed that the average person knows how to count to four, and therefore is capable of determining the number of hydrogen atoms even though they are not shown. So you only need to count the number of bonds that you can see on a carbon atom, and then you know that there should be enough hydrogen atoms to give a total of four bonds to the carbon atom. After doing this many times, you will get to a point where you do not need to count anymore. You will simply get accustomed to seeing these types of drawings, and you will be able to instantly “see” all of the hydrogen atoms without counting them. Now we will do some exercises that will help you get to that point. O We see only two bonds connected to this carbon atom 1.1 HOW TO READ BOND-LINE DRAWINGS 3 O O OH N H O O OH 1.2 Answer: 1.3 Answer: 1.4 Answer: 1.5 Answer: 1.6 Answer: 1.7 Answer: O 1.8 Answer: 1.9 Answer: 1.11 Answer: O 1.10 Answer: 6753_Klein_01.qxd 5/1/07 5:03 PM Page 3