正在加载图片...
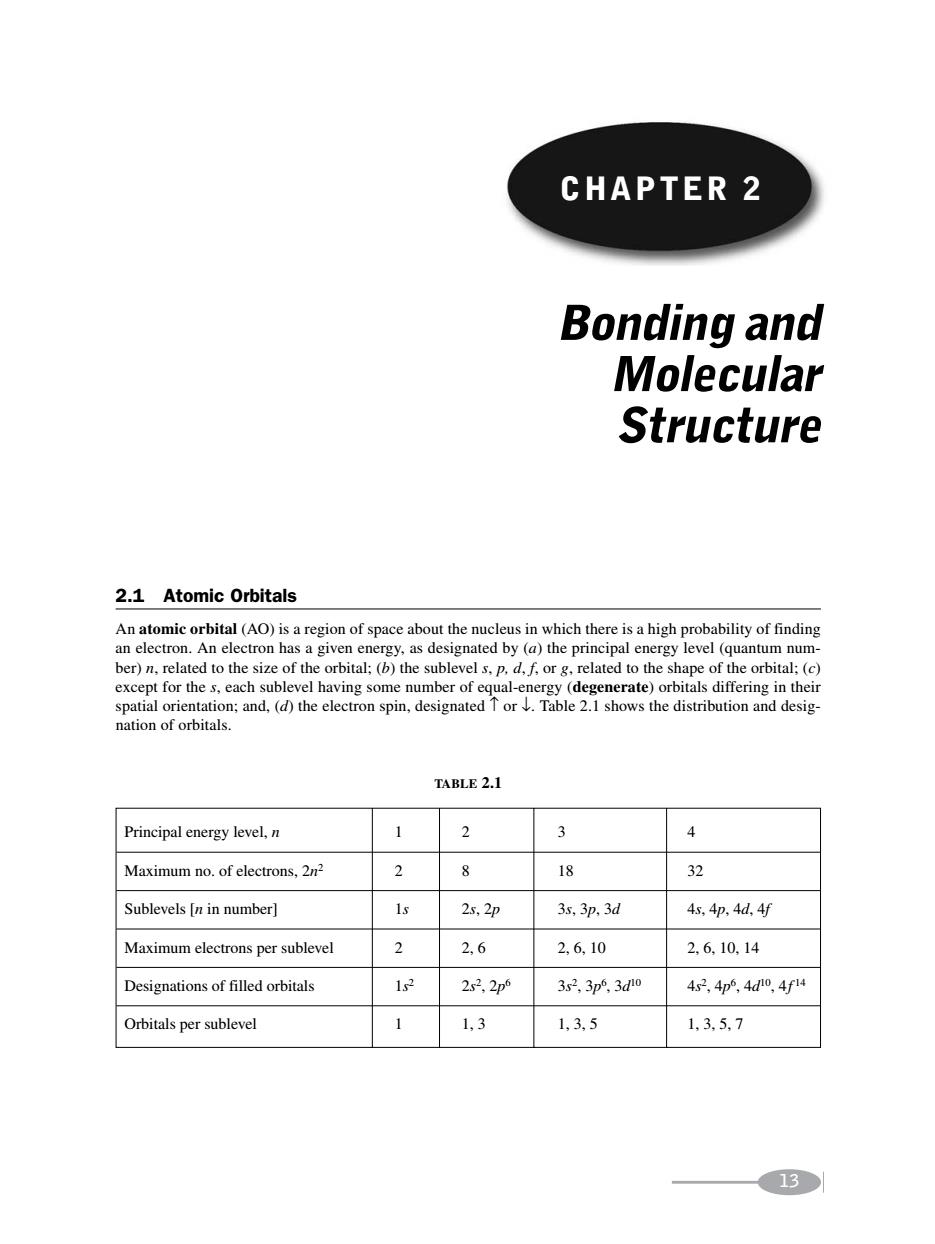
CHAPTER 2 Bonding and Molecular Structure 2.1 Atomic Orbitals e pr energ aeobae except for the s,each sublevel having some number of e al-en renerate)orbitals differing in their spatial orientation:and.(d)the electron spin.designatedor Table2.1 shows the distribution and desig- nation of orbitals. TABLE2.1 Principal energy level,n 3 Maximum no.of electrons,2m 2 8 18 32 Sublevels [n in number] 2s.2p 3x,3p,3d 4s.4p.4d.45 Maximum electrons per sublevel 26 2,6,10 2.6.10.14 Designations of filled orbitals 1s2 2x32p 3r23p3d0 4s2.4p4d,4f4 Orbitals per sublevel 1,3 1,3,5 1,35.7 13CHAPTER 2 CHAPTER 2 13 Bonding and Molecular Structure 2.1 Atomic Orbitals An atomic orbital (AO) is a region of space about the nucleus in which there is a high probability of finding an electron. An electron has a given energy, as designated by (a) the principal energy level (quantum number) n, related to the size of the orbital; (b) the sublevel s, p, d, f, or g, related to the shape of the orbital; (c) except for the s, each sublevel having some number of equal-energy (degenerate) orbitals differing in their spatial orientation; and, (d) the electron spin, designated ↑ or ↓. Table 2.1 shows the distribution and designation of orbitals. TABLE 2.1 Principal energy level, n 12 3 4 Maximum no. of electrons, 2n2 2 8 18 32 Sublevels [n in number] 1s 2s, 2p 3s, 3p, 3d 4s, 4p, 4d, 4f Maximum electrons per sublevel 2 2, 6 2, 6, 10 2, 6, 10, 14 Designations of filled orbitals 1s2 2s2, 2p6 3s2, 3p6 , 3d10 4s2, 4p6, 4d10, 4f 14 Orbitals per sublevel 1 1, 3 1, 3, 5 1, 3, 5, 7