正在加载图片...
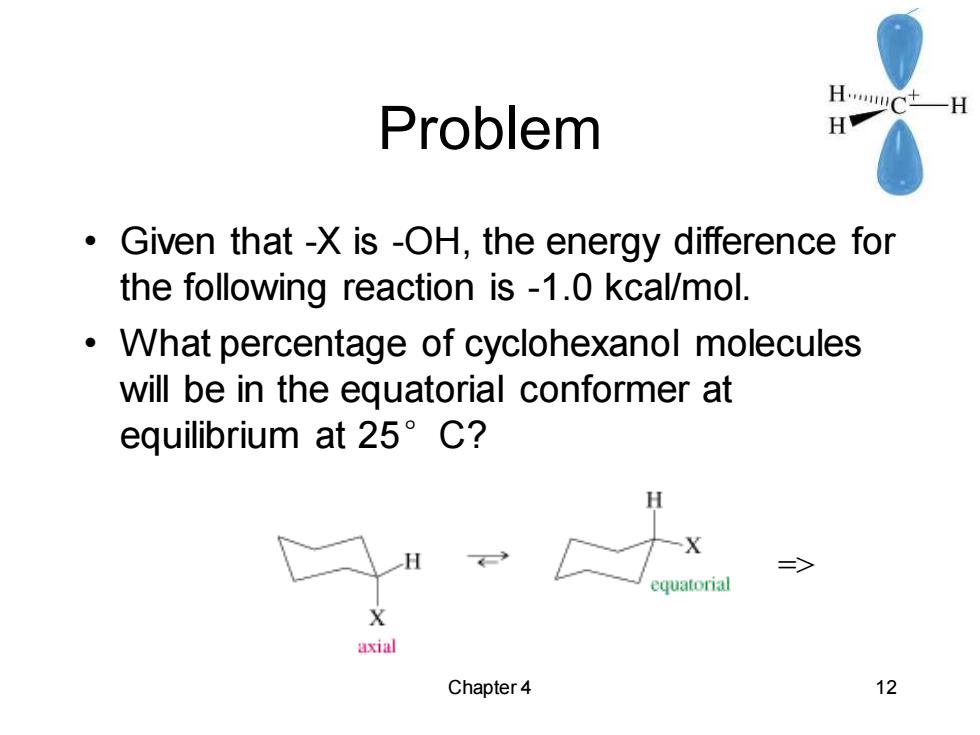
Problem HwCtH H Given that -X is -OH,the energy difference for the following reaction is -1.0 kcal/mol. What percentage of cyclohexanol molecules will be in the equatorial conformer at equilibrium at25°C? H => equatorial X axial Chapter4 12Chapter 4 12 Problem • Given that -X is -OH, the energy difference for the following reaction is -1.0 kcal/mol. • What percentage of cyclohexanol molecules will be in the equatorial conformer at equilibrium at 25°C? =>