正在加载图片...
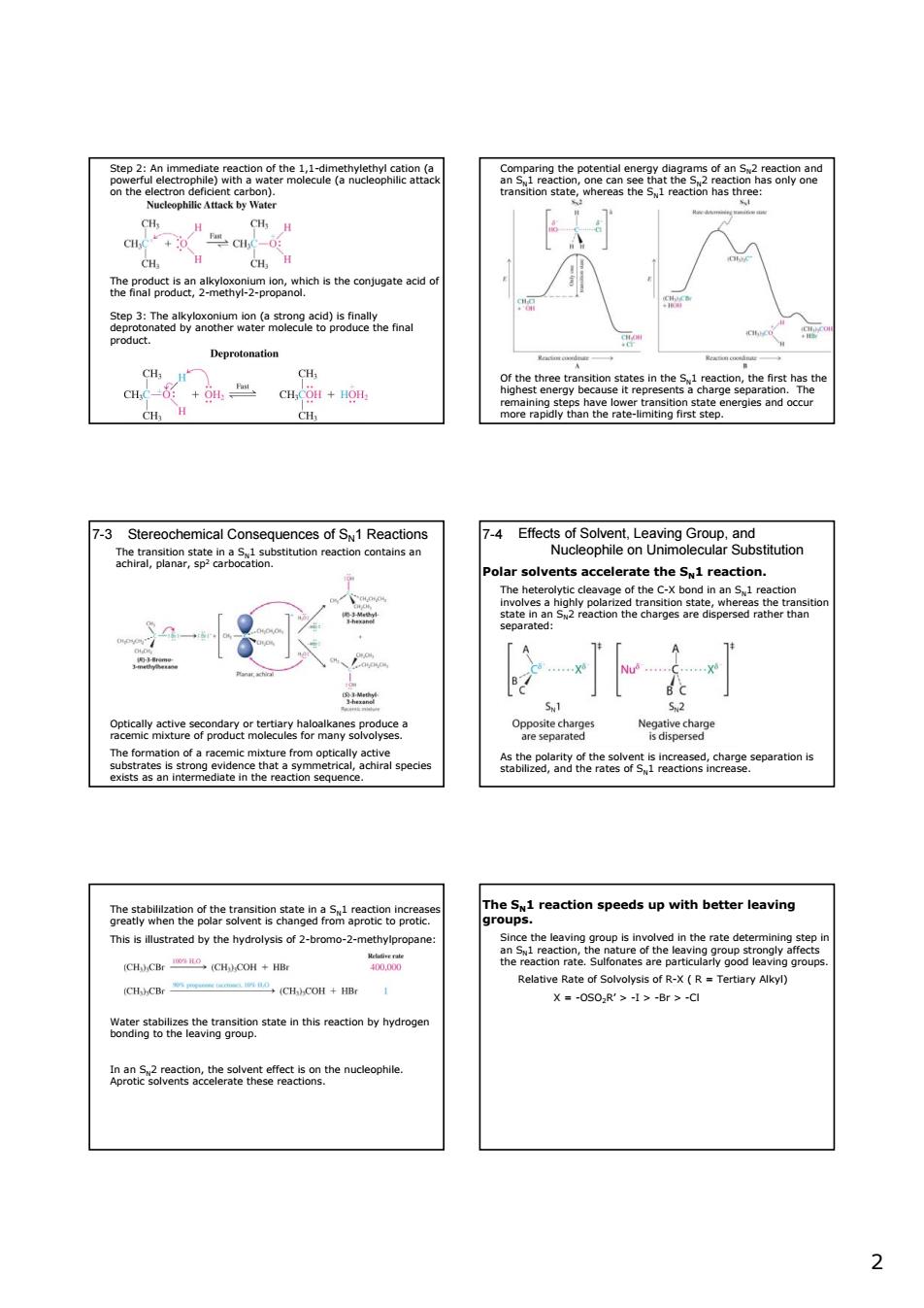
股Sbea2nanateconwoaea .w Deprotonation of the n the 5.1 re +40 CH H 7-3 Stereochemical Consequences of SN1 Reactions stitution reaction contains ar 4Eeeoeeoem8r8shion C-X 可 9oaaatgenoaczoraarhooawygoneae g e formation of a 8egg88252epa6ons ction speeds up with better leaving rolysis of 2-bromo GHC巴OH+H Relattve Rate at ot B-xtB=T CH CBr CH.)COH+ X=-0s0R'>-1>-Br>-Ca statethsco byoe th nucleophi 22 Step 2: An immediate reaction of the 1,1-dimethylethyl cation (a powerful electrophile) with a water molecule (a nucleophilic attack on the electron deficient carbon). The product is an alkyloxonium ion, which is the conjugate acid of the final product, 2-methyl-2-propanol. Step 3: The alkyloxonium ion (a strong acid) is finally deprotonated by another water molecule to produce the final product. Comparing the potential energy diagrams of an SN2 reaction and an SN1 reaction, one can see that the SN2 reaction has only one transition state, whereas the SN1 reaction has three: Of the three transition states in the SN1 reaction, the first has the highest energy because it represents a charge separation. The remaining steps have lower transition state energies and occur more rapidly than the rate-limiting first step. 7-3 Stereochemical Consequences of SN1 Reactions Optically active secondary or tertiary haloalkanes produce a racemic mixture of product molecules for many solvolyses. The formation of a racemic mixture from optically active substrates is strong evidence that a symmetrical, achiral species exists as an intermediate in the reaction sequence. The transition state in a SN1 substitution reaction contains an achiral, planar, sp2 carbocation. Effects of Solvent, Leaving Group, and Nucleophile on Unimolecular Substitution 7-4 Polar solvents accelerate the SN1 reaction. The heterolytic cleavage of the C-X bond in an SN1 reaction involves a highly polarized transition state, whereas the transition state in an SN2 reaction the charges are dispersed rather than separated: As the polarity of the solvent is increased, charge separation is stabilized, and the rates of SN1 reactions increase. The stabililzation of the transition state in a SN1 reaction increases greatly when the polar solvent is changed from aprotic to protic. This is illustrated by the hydrolysis of 2-bromo-2-methylpropane: Water stabilizes the transition state in this reaction by hydrogen bonding to the leaving group. In an SN2 reaction, the solvent effect is on the nucleophile. Aprotic solvents accelerate these reactions. The SN1 reaction speeds up with better leaving groups. Since the leaving group is involved in the rate determining step in an SN1 reaction, the nature of the leaving group strongly affects the reaction rate. Sulfonates are particularly good leaving groups. Relative Rate of Solvolysis of R-X ( R = Tertiary Alkyl) X = -OSO2R’ > -I > -Br > -Cl