正在加载图片...
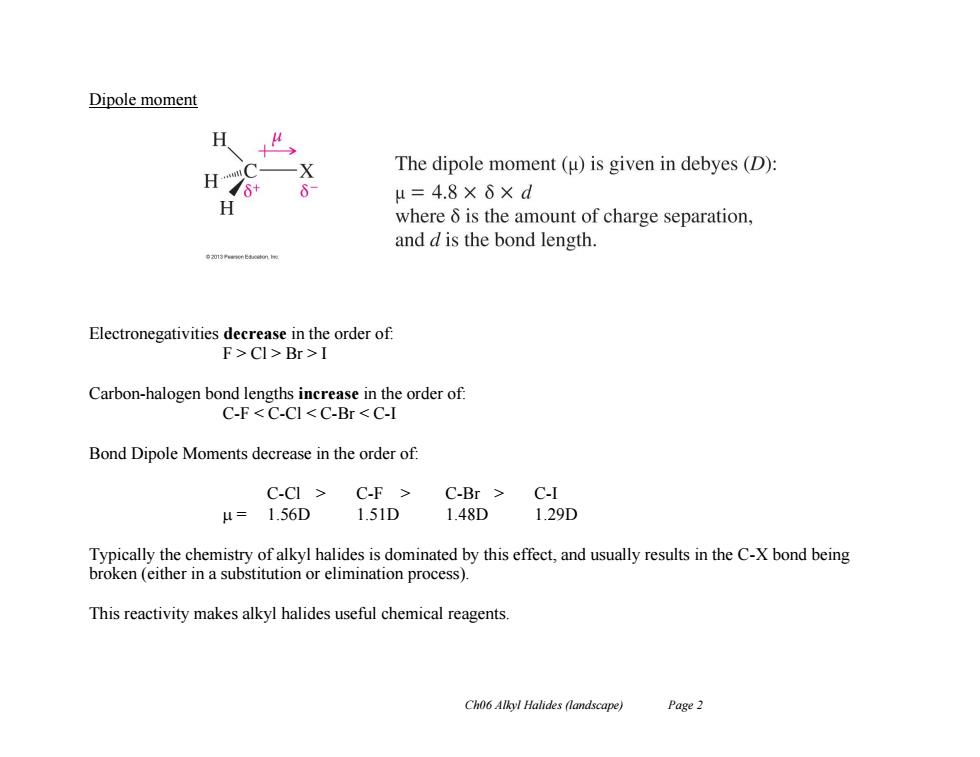
Dipole moment 夕 The dipole moment(u)is given in debyes(D): H7* μ=4.8×6×d where 6 is the amount of charge separation, and d is the bond length. Electronegativities decrease in the order of: F>Cl>Br>I Carbon-halogen bond lengths increase in the order of: C-F<C-CI<C-Br<C-I Bond Dipole Moments decrease in the order of: C-CI C-F C-Br C-I μ=1.56D 1.51D1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect,and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents Ch06 Alkyl Halides (landscape) Page 2 Ch06 Alkyl Halides (landscape) Page 2 Dipole moment Electronegativities decrease in the order of: F > Cl > Br > I Carbon-halogen bond lengths increase in the order of: C-F < C-Cl < C-Br < C-I Bond Dipole Moments decrease in the order of: C-Cl > C-F > C-Br > C-I = 1.56D 1.51D 1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect, and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents