正在加载图片...
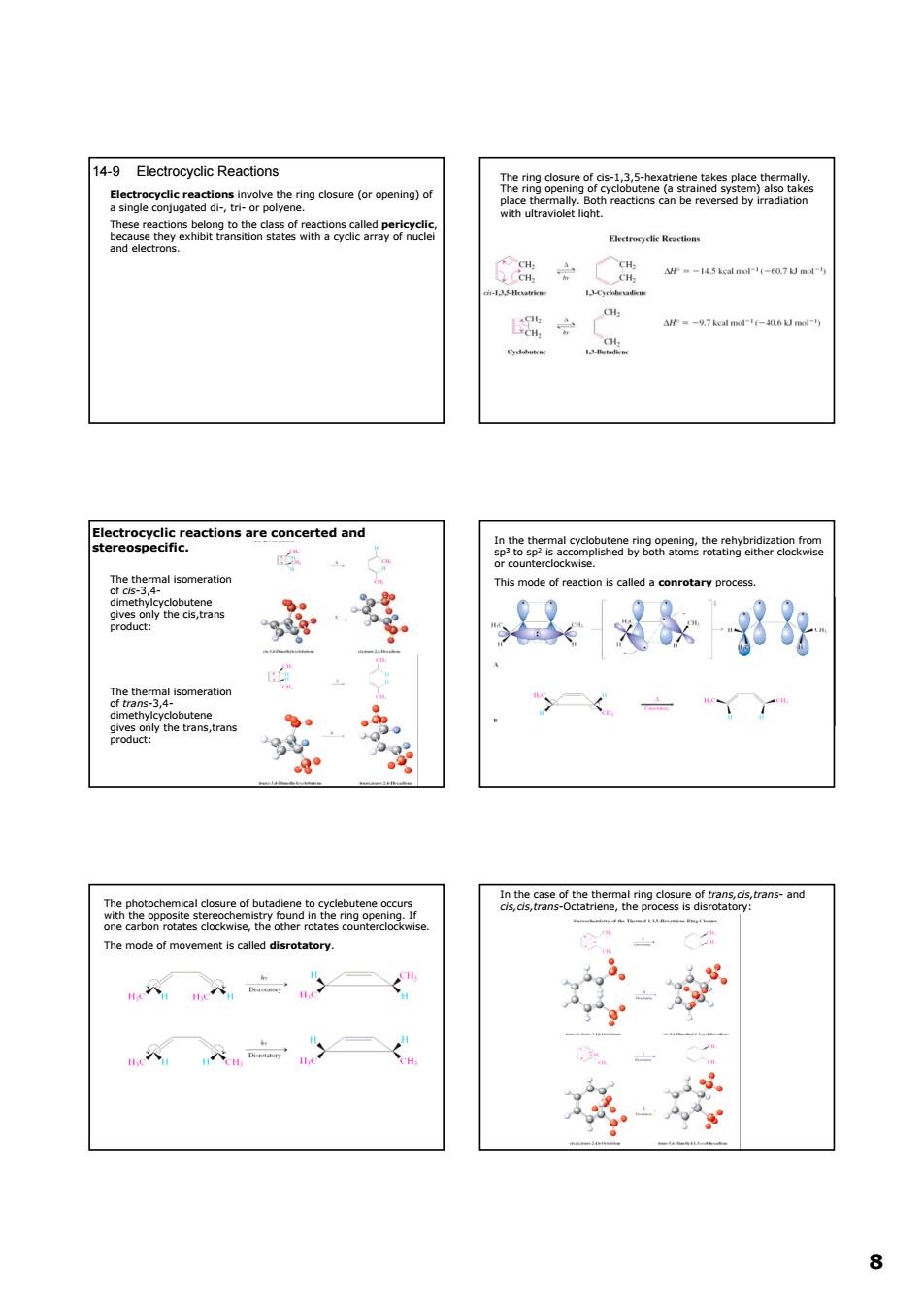
14-9 Electrocyclic Reactions &5nge2gtatntoetetgdosure(oroponng)o oR机g2 3 具为x38好 aaebe2lep2o8s68lra6as.ans-and The mode of movements clled disroaory. 88 14-9 Electrocyclic Reactions Electrocyclic reactions involve the ring closure (or opening) of a single conjugated di-, tri- or polyene. These reactions belong to the class of reactions called pericyclic, because they exhibit transition states with a cyclic array of nuclei and electrons. The ring closure of cis-1,3,5-hexatriene takes place thermally. The ring opening of cyclobutene (a strained system) also takes place thermally. Both reactions can be reversed by irradiation with ultraviolet light. Electrocyclic reactions are concerted and stereospecific. The thermal isomeration of cis-3,4- dimethylcyclobutene gives only the cis,trans product: The thermal isomeration of trans-3,4- dimethylcyclobutene gives only the trans,trans product: In the thermal cyclobutene ring opening, the rehybridization from sp3 to sp2 is accomplished by both atoms rotating either clockwise or counterclockwise. This mode of reaction is called a conrotary process. The photochemical closure of butadiene to cyclebutene occurs with the opposite stereochemistry found in the ring opening. If one carbon rotates clockwise, the other rotates counterclockwise. The mode of movement is called disrotatory. In the case of the thermal ring closure of trans,cis,trans- and cis,cis,trans-Octatriene, the process is disrotatory: