正在加载图片...
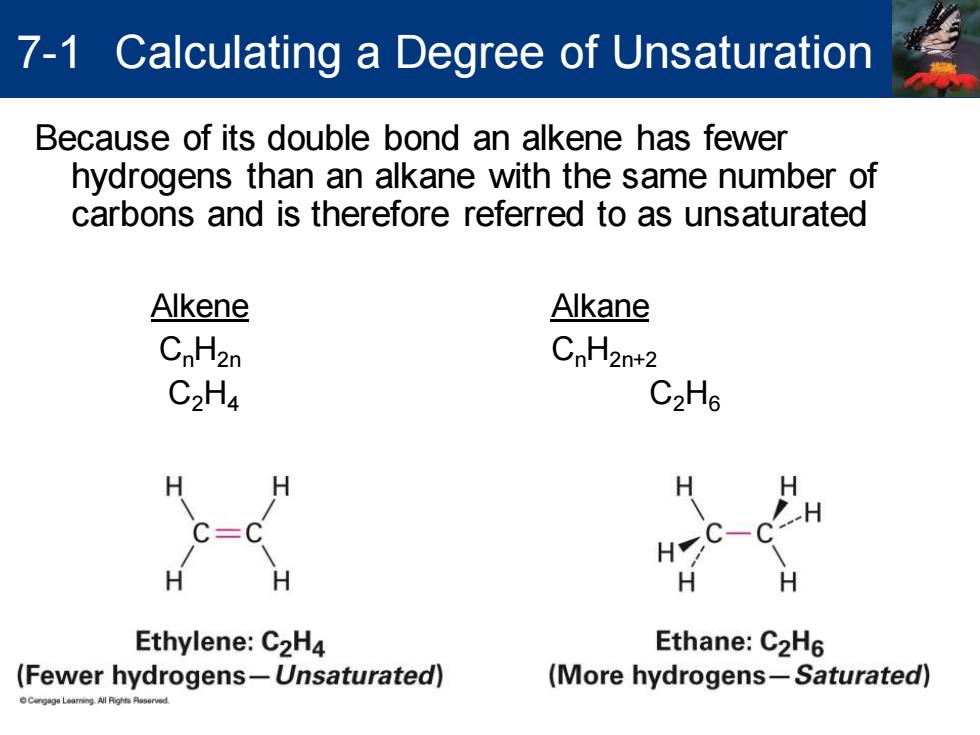
7-1 Calculating a Degree of Unsaturation Because of its double bond an alkene has fewer hydrogens than an alkane with the same number of carbons and is therefore referred to as unsaturated Alkene Alkane CnH2n CnH2n+2 C2H4 C2H6 H Ethylene:C2H4 Ethane:C2H6 (Fewer hydrogens-Unsaturated) (More hydrogens-Saturated) Because of its double bond an alkene has fewer hydrogens than an alkane with the same number of carbons and is therefore referred to as unsaturated Alkene Alkane CnH2n CnH2n+2 C2H4 C2H6 7-1 Calculating a Degree of Unsaturation