正在加载图片...
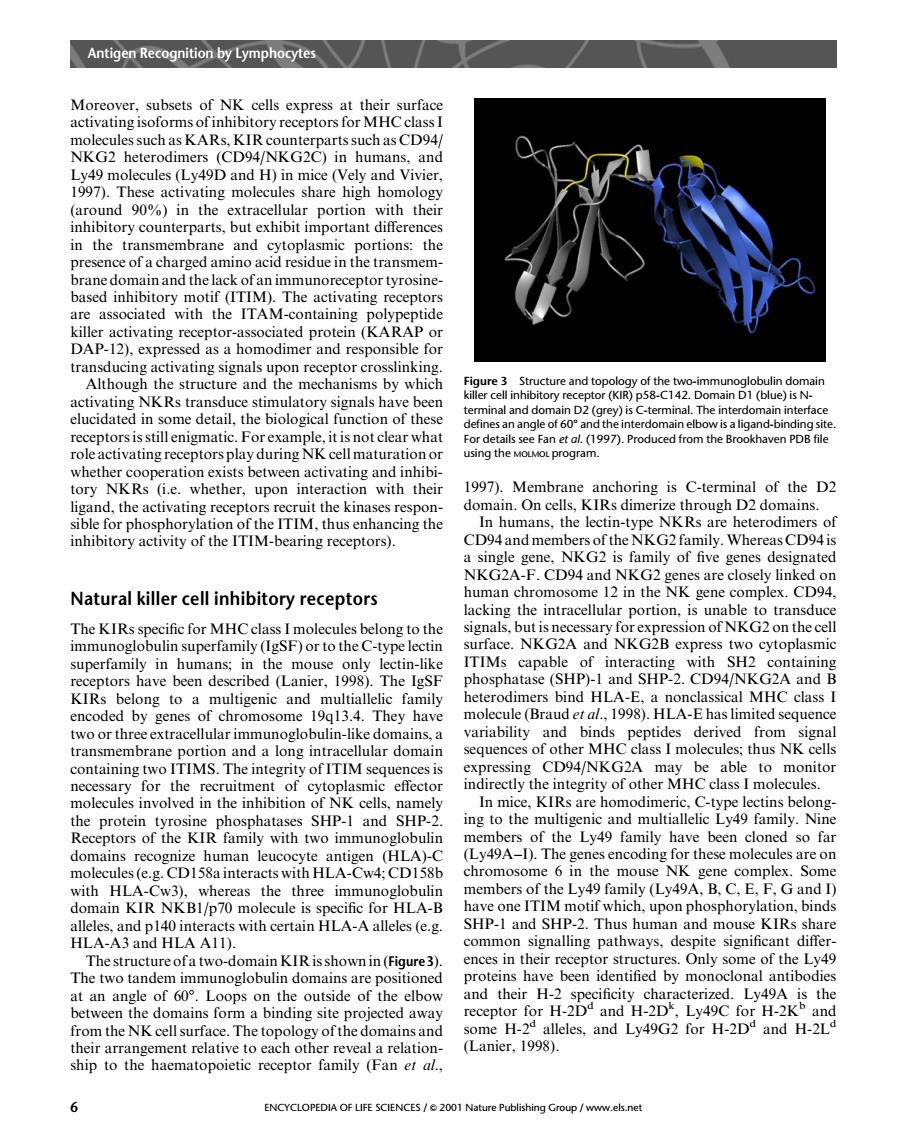
Antigymphye Moreover,subsets of NK cells express at their surface activating isoforms of inhibitory receptors for MHCclass nd H)i 1997)These act ivating molecules share high homolo (around 90%)in the extracellular portion with thei inhibitory counterparts,but exhibit important difference in the the brane domain and the lack of an immunoreceptor tyrosine ass ate the ITA DAP-12 transducing activating signals upon receptor crosslinking Although the structure and the mechanisms by which me t 7heaigand-bine role activating receptors play during NK cell maturation or using the Moo program. aven PD whethe ory whethe upon with their 997)M choring is C-terminal of the D2 In humans,the lectin-type NKRs are heterodimers of inhibitory activity of the ITIM-bearing receptors). CD94and members of the NKG2 family.Whereas CD94is mily of fve genes designate Natural killer cell inhibitory receptors nan chr 12 in the NK CD94 lacking the intracellular portion,is unable to transduc The KIRs specific for MHCclass I molecules belong to the immunoglobulin superfamily (IgSF)or to the C-type lectin TIMs expre cytop superfamily in humans;in ed hatase (SHP)-1 and SHP-2.CD94/NKG2A and B heterodimers bind HLA-E,a nonclassical MHC class I encoded by genes of chromosome 19913.4.They have molecule(Brauder al.,1998).HLA-E has limited sequenc and transh 1g in CD94/NKG2A abl n for the asmic indirectly the integrity of other MHC class I molecules. molecules involved in the inhibition of NK cells,namely SHP-1 and SHP-2 amily (Ly49A-D.The the se mo ecules chromosome 6 in the mouse NK gene complex Some with HLA-( Cw3).whereas the three immunoglobuli members of L491 d HLA-B spnory HLA-A3AD HLAALD eles (e.g malling nathways ain KIR isshown in(Fig ences in their receptor structures.Only some of the Ly49 The two tandem immunoglobulin domains are positioned teins hav been identified by monoclona antibodies an angle of 60 the ou side of the elbow and their ng site ected awa c. rH-2D nd H-2D H-2k the alleles.and Lv49G2 for H-2D and H-2L eal a relation (Lanier.1998) ship to the haematopoietic receptor family (Fan et al. 6 ENCYCLOPEDIA OF LIFE SCIENCES/2001 els neMoreover, subsets of NK cells express at their surface activating isoforms of inhibitory receptors for MHC class I molecules such as KARs, KIR counterparts such as CD94/ NKG2 heterodimers (CD94/NKG2C) in humans, and Ly49 molecules (Ly49D and H) in mice (Vely and Vivier, 1997). These activating molecules share high homology (around 90%) in the extracellular portion with their inhibitory counterparts, but exhibit important differences in the transmembrane and cytoplasmic portions: the presence of a charged amino acid residue in the transmembrane domain and the lack of an immunoreceptor tyrosinebased inhibitory motif (ITIM). The activating receptors are associated with the ITAM-containing polypeptide killer activating receptor-associated protein (KARAP or DAP-12), expressed as a homodimer and responsible for transducing activating signals upon receptor crosslinking. Although the structure and the mechanisms by which activating NKRs transduce stimulatory signals have been elucidated in some detail, the biological function of these receptors is still enigmatic. For example, it is not clear what role activating receptors play during NK cell maturation or whether cooperation exists between activating and inhibitory NKRs (i.e. whether, upon interaction with their ligand, the activating receptors recruit the kinases responsible for phosphorylation of the ITIM, thus enhancing the inhibitory activity of the ITIM-bearing receptors). Natural killer cell inhibitory receptors The KIRs specific for MHC class I molecules belong to the immunoglobulin superfamily (IgSF) or to the C-type lectin superfamily in humans; in the mouse only lectin-like receptors have been described (Lanier, 1998). The IgSF KIRs belong to a multigenic and multiallelic family encoded by genes of chromosome 19q13.4. They have two or three extracellular immunoglobulin-like domains, a transmembrane portion and a long intracellular domain containing two ITIMS. The integrity of ITIM sequences is necessary for the recruitment of cytoplasmic effector molecules involved in the inhibition of NK cells, namely the protein tyrosine phosphatases SHP-1 and SHP-2. Receptors of the KIR family with two immunoglobulin domains recognize human leucocyte antigen (HLA)-C molecules (e.g. CD158a interacts with HLA-Cw4; CD158b with HLA-Cw3), whereas the three immunoglobulin domain KIR NKB1/p70 molecule is specific for HLA-B alleles, and p140 interacts with certain HLA-A alleles (e.g. HLA-A3 and HLA A11). The structure of a two-domain KIR is shown in (Figure3). The two tandem immunoglobulin domains are positioned at an angle of 608. Loops on the outside of the elbow between the domains form a binding site projected away from the NK cell surface. The topology of the domains and their arrangement relative to each other reveal a relationship to the haematopoietic receptor family (Fan et al., 1997). Membrane anchoring is C-terminal of the D2 domain. On cells, KIRs dimerize through D2 domains. In humans, the lectin-type NKRs are heterodimers of CD94 and members of the NKG2 family.Whereas CD94 is a single gene, NKG2 is family of five genes designated NKG2A-F. CD94 and NKG2 genes are closely linked on human chromosome 12 in the NK gene complex. CD94, lacking the intracellular portion, is unable to transduce signals, but is necessary for expression of NKG2 on the cell surface. NKG2A and NKG2B express two cytoplasmic ITIMs capable of interacting with SH2 containing phosphatase (SHP)-1 and SHP-2. CD94/NKG2A and B heterodimers bind HLA-E, a nonclassical MHC class I molecule (Braud et al., 1998). HLA-E has limited sequence variability and binds peptides derived from signal sequences of other MHC class I molecules; thus NK cells expressing CD94/NKG2A may be able to monitor indirectly the integrity of other MHC class I molecules. In mice, KIRs are homodimeric, C-type lectins belonging to the multigenic and multiallelic Ly49 family. Nine members of the Ly49 family have been cloned so far (Ly49A–I). The genes encoding for these molecules are on chromosome 6 in the mouse NK gene complex. Some members of the Ly49 family (Ly49A, B, C, E, F, G and I) have one ITIM motif which, upon phosphorylation, binds SHP-1 and SHP-2. Thus human and mouse KIRs share common signalling pathways, despite significant differences in their receptor structures. Only some of the Ly49 proteins have been identified by monoclonal antibodies and their H-2 specificity characterized. Ly49A is the receptor for H-2Dd and H-2Dk , Ly49C for H-2Kb and some H-2d alleles, and Ly49G2 for H-2Dd and H-2Ld (Lanier, 1998). Figure 3 Structure and topology of the two-immunoglobulin domain killer cell inhibitory receptor (KIR) p58-C142. Domain D1 (blue) is Nterminal and domain D2 (grey) is C-terminal. The interdomain interface defines an angle of 608 and the interdomain elbow is a ligand-binding site. For details see Fan et al. (1997). Produced from the Brookhaven PDB file using the MOLMOL program. Antigen Recognition by Lymphocytes 6 ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net