正在加载图片...
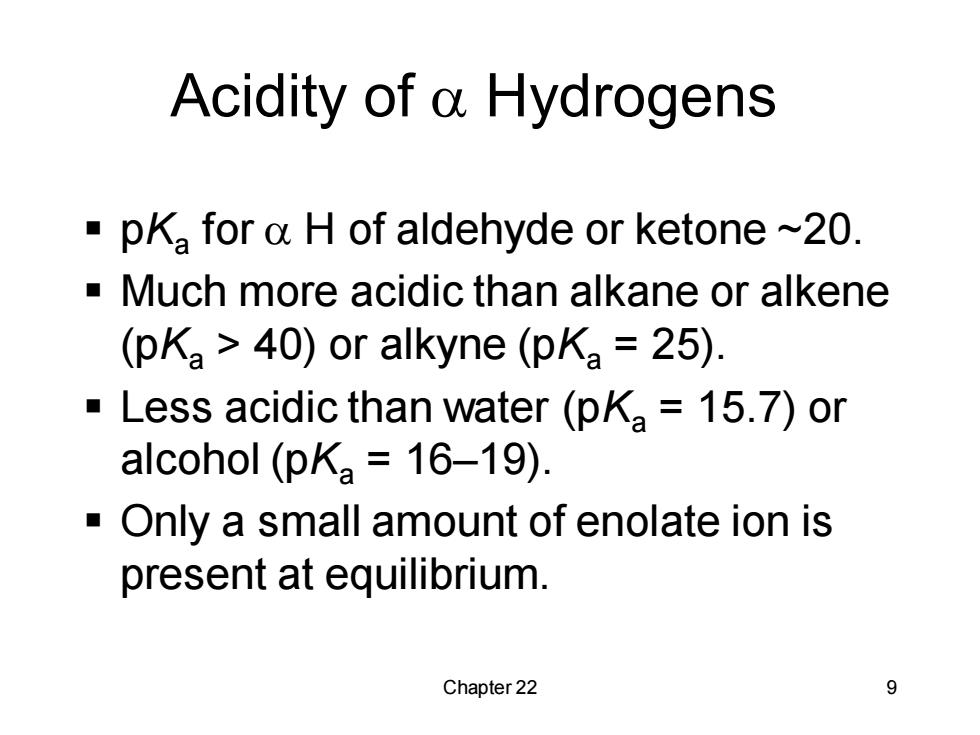
Acidity of a Hydrogens pKa for a H of aldehyde or ketone ~20. Much more acidic than alkane or alkene (pKa 40)or alkyne(pKa 25). Less acidic than water (pKa 15.7)or alcohol (pKa 16-19). Only a small amount of enolate ion is present at equilibrium. Chapter 22 9Chapter 22 9 Acidity of a Hydrogens ▪ pKa for a H of aldehyde or ketone ~20. ▪ Much more acidic than alkane or alkene (pKa > 40) or alkyne (pKa = 25). ▪ Less acidic than water (pKa = 15.7) or alcohol (pKa = 16–19). ▪ Only a small amount of enolate ion is present at equilibrium