正在加载图片...
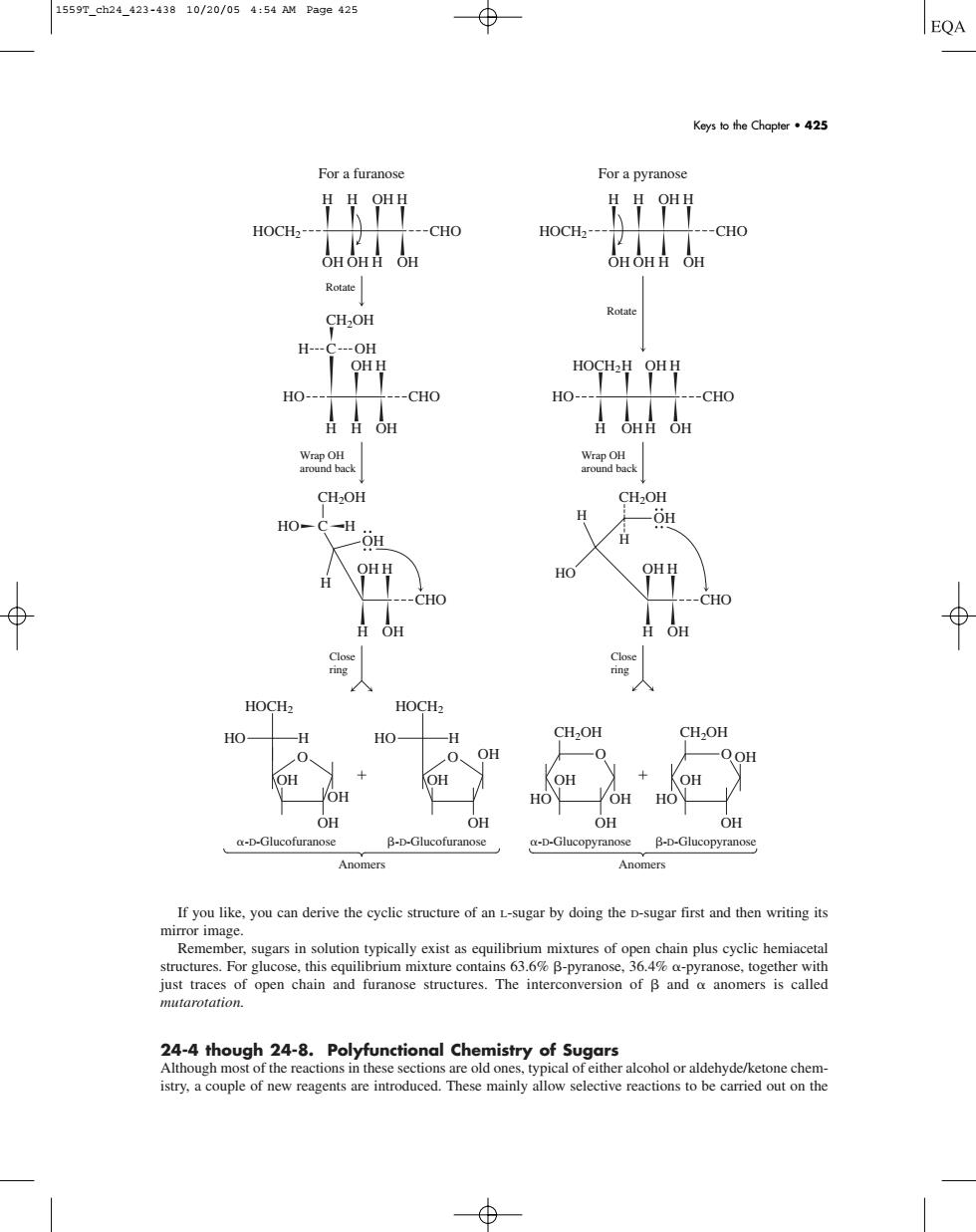
1559T_ch24_423-43810/20/054:54 AM Page425 EQA Keys to the Chapter·425 For a furanose For a pyranose HOCH2 --CHO HOCH2 --CHO H--C---OH HOCHH OHH HO- --CHO HO- --CHO 日0n日0 CH2OH HO-C-H 9 CHO CHO 人 HOCH CH,OH CH2OH OH DOH OH OH OH OH HO B-D-Glucof a-D-Glu B-D-Glu If you like.you can derive the cyclic structure of an L-sugar by doing the D-sugar first and then writing its ether with just traces of open chain and furanose structures.The interconversion of B and anomers is called mutarotation. 24-4 tho e old ond istry,a couple of new reagents are introduced.These mainly allow selective reactions to be carried out on the If you like, you can derive the cyclic structure of an L-sugar by doing the D-sugar first and then writing its mirror image. Remember, sugars in solution typically exist as equilibrium mixtures of open chain plus cyclic hemiacetal structures. For glucose, this equilibrium mixture contains 63.6% -pyranose, 36.4% -pyranose, together with just traces of open chain and furanose structures. The interconversion of and anomers is called mutarotation. 24-4 though 24-8. Polyfunctional Chemistry of Sugars Although most of the reactions in these sections are old ones, typical of either alcohol or aldehyde/ketone chemistry, a couple of new reagents are introduced. These mainly allow selective reactions to be carried out on the For a furanose For a pyranose OH OH -D-Glucofuranose -D-Glucopyranose OH O CH2OH HO H CH2OH C H OH HO CH2OH C Rotate HO Wrap OH around back Wrap OH around back Close ring Close ring Rotate H OH CH2OH H H HO OH OH OH -D-Glucofuranose Anomers Anomers OH O OH OH OH O -D-Glucopyranose CH2OH HO OH OH OH O HOCH2 CHO H H OH H OH OH H OH HOCH2 CHO H H OH H OH OH H OH HO CHO OH H H H OH HOCH2 CHO H OH H H OHH OH CHO OH H H OH CHO OH H H OH HOCH2 HO H HOCH2 HO H Keys to the Chapter • 425 1559T_ch24_423-438 10/20/05 4:54 AM Page 425������