正在加载图片...
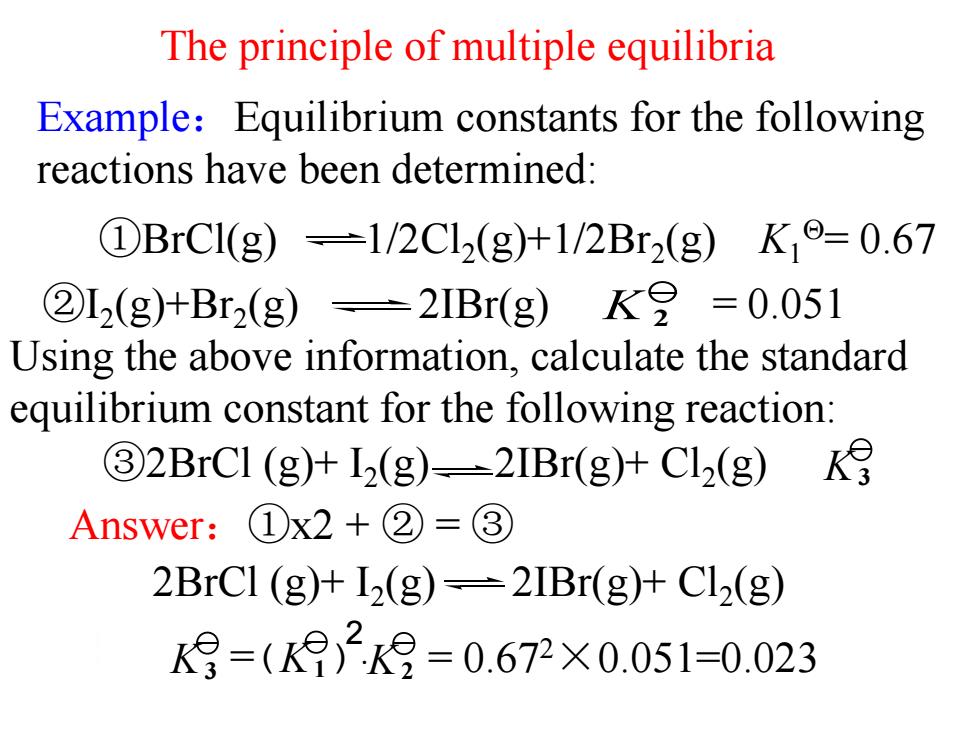
The principle of multiple equilibria Example:Equilibrium constants for the following reactions have been determined: ①BrC1(g)=1/2Cl2(g)+1/2Br2(g)K,o=0.67 ②L2(g)+Br2(g)=2IBr(g)K8=0.051 Using the above information,calculate the standard equilibrium constant for the following reaction: 32BrCI (g)+12(g)-2IBr(g)+Cl2(g) Answer:①x2+②=③ 2BrCl (g)+12(g)=2IBr(g)+Cl2(g) K月=()29=0.672X0.051=0.023 Example:Equilibrium constants for the following reactions have been determined: The principle of multiple equilibria Answer:①x2 + ② = ③ K 2 ②I2 (g)+Br2 (g) 2IBr(g) = 0.051 Using the above information, calculate the standard equilibrium constant for the following reaction: ①BrCl(g) 1/2Cl2 (g)+1/2Br2 (g) K1 = 0.67 2BrCl (g)+ I2 (g) 2IBr(g)+ Cl2 (g) ③2BrCl (g)+ I2 (g) 2IBr(g)+ Cl2 (g) K3 = · = 0.67 K3 K1 K2 2×0.051=0.023 2 ( )