正在加载图片...
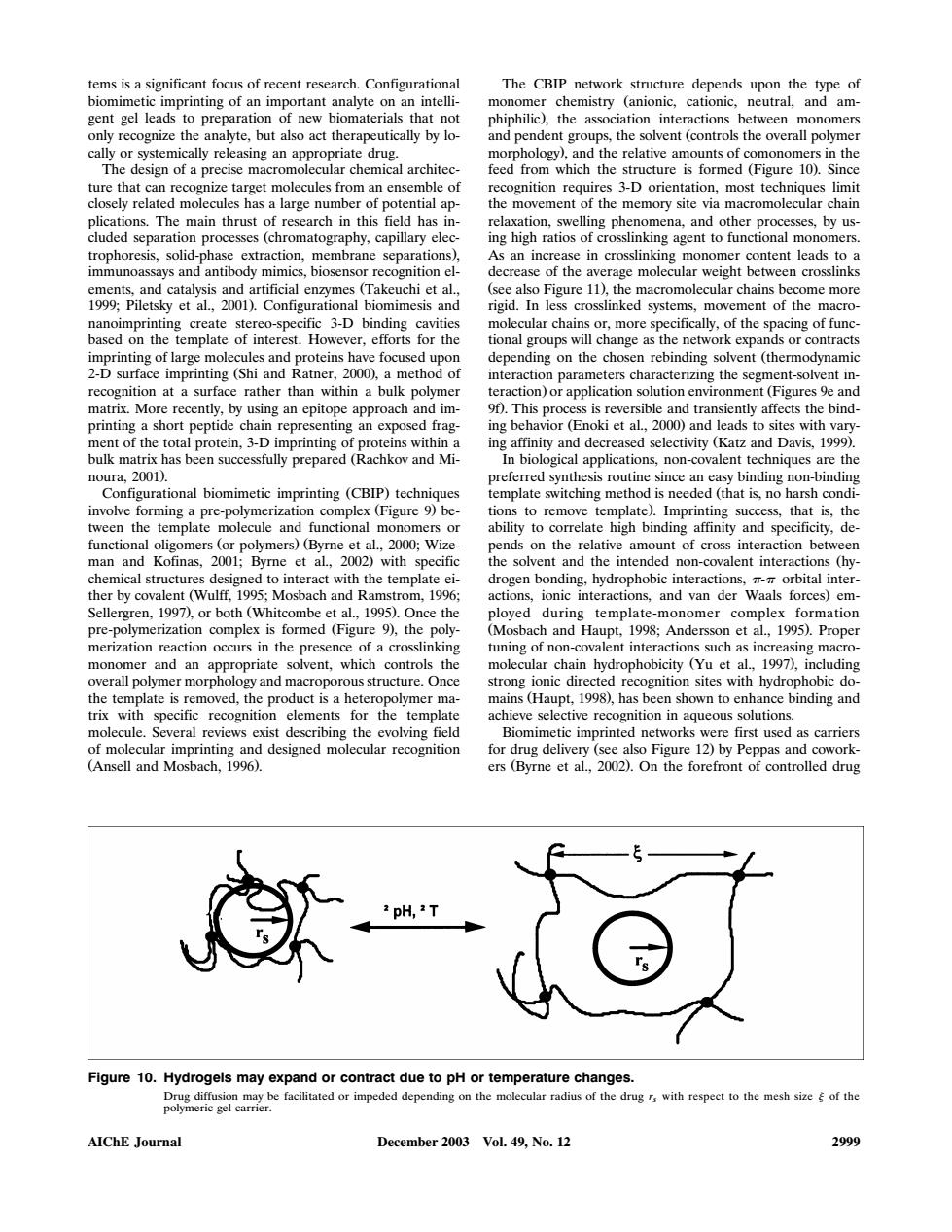
tems is a significant focus of recent research.Configurationa The CBIP network structure depends upon the type of s the solvent (controls the overall polyme n the ture that ing high ratios of cr gnition el erage molecular weight aa20dartc chains be ome more imprinting ic 3-D bir lecular chain or inte nd and raction para using an ep oach and im ap s is rex hle and transie ntly affects the bind- repr famictl2noand ds to s with vary biomimetie(CBP)technique eonpre-polyme z: lons to mov 55. that nal 2000:Wiz ds on the r man and Kofinas. et al wth ent and the intende non-covalent inter. by covalent (Wulff, 199 ach and ra 199 and van der 997.d hitcombe o 9)the reaction rs in the pres all nition site phobicdo product is a h wn binding and lecule Several rev evolving field mimetic impr inted nety first used s carrier rug al,2002.0n- ppas an 一© Figure 10.Hydrogels may expand or contract due to pH or temperature changes. r impeded depending on the molecular radius of the drug r with respect to the mesh size of the AIChE Journal December 2003 Vol.49.No.12 2999tems is a significant focus of recent research. Configurational biomimetic imprinting of an important analyte on an intelligent gel leads to preparation of new biomaterials that not only recognize the analyte, but also act therapeutically by locally or systemically releasing an appropriate drug. The design of a precise macromolecular chemical architecture that can recognize target molecules from an ensemble of closely related molecules has a large number of potential applications. The main thrust of research in this field has included separation processes chromatography, capillary elec- Ž trophoresis, solid-phase extraction, membrane separations ,. immunoassays and antibody mimics, biosensor recognition elements, and catalysis and artificial enzymes Takeuchi et al., Ž 1999; Piletsky et al., 2001 . Configurational biomimesis and . nanoimprinting create stereo-specific 3-D binding cavities based on the template of interest. However, efforts for the imprinting of large molecules and proteins have focused upon 2-D surface imprinting Shi and Ratner, 2000 , a method of Ž . recognition at a surface rather than within a bulk polymer matrix. More recently, by using an epitope approach and imprinting a short peptide chain representing an exposed fragment of the total protein, 3-D imprinting of proteins within a bulk matrix has been successfully prepared Rachkov and Mi- Ž noura, 2001 .. Configurational biomimetic imprinting CBIP techniques Ž . involve forming a pre-polymerization complex Figure 9 be- Ž . tween the template molecule and functional monomers or functional oligomers or polymers Byrne et al., 2000; Wize- Ž .Ž man and Kofinas, 2001; Byrne et al., 2002 with specific . chemical structures designed to interact with the template either by covalent Wulff, 1995; Mosbach and Ramstrom, 1996; Ž Sellergren, 1997 , or both Whitcombe et al., 1995 . Once the .Ž . pre-polymerization complex is formed Figure 9 , the poly- Ž . merization reaction occurs in the presence of a crosslinking monomer and an appropriate solvent, which controls the overall polymer morphology and macroporous structure. Once the template is removed, the product is a heteropolymer matrix with specific recognition elements for the template molecule. Several reviews exist describing the evolving field of molecular imprinting and designed molecular recognition Ž . Ansell and Mosbach, 1996 . The CBIP network structure depends upon the type of monomer chemistry anionic, cationic, neutral, and am- Ž phiphilic , the association interactions between monomers . and pendent groups, the solvent controls the overall polymer Ž morphology , and the relative amounts of comonomers in the . feed from which the structure is formed Figure 10 . Since Ž . recognition requires 3-D orientation, most techniques limit the movement of the memory site via macromolecular chain relaxation, swelling phenomena, and other processes, by using high ratios of crosslinking agent to functional monomers. As an increase in crosslinking monomer content leads to a decrease of the average molecular weight between crosslinks Ž . see also Figure 11 , the macromolecular chains become more rigid. In less crosslinked systems, movement of the macromolecular chains or, more specifically, of the spacing of functional groups will change as the network expands or contracts depending on the chosen rebinding solvent thermodynamic Ž interaction parameters characterizing the segment-solvent interaction or application solution environment Figures 9e and . Ž 9f . This process is reversible and transiently affects the bind- . ing behavior Enoki et al., 2000 and leads to sites with vary- Ž . ing affinity and decreased selectivity Katz and Davis, 1999 . Ž . In biological applications, non-covalent techniques are the preferred synthesis routine since an easy binding non-binding template switching method is needed that is, no harsh condi- Ž tions to remove template . Imprinting success, that is, the . ability to correlate high binding affinity and specificity, depends on the relative amount of cross interaction between the solvent and the intended non-covalent interactions hy- Ž drogen bonding, hydrophobic interactions, - orbital interactions, ionic interactions, and van der Waals forces em- . ployed during template-monomer complex formation Ž . Mosbach and Haupt, 1998; Andersson et al., 1995 . Proper tuning of non-covalent interactions such as increasing macromolecular chain hydrophobicity Yu et al., 1997 , including Ž . strong ionic directed recognition sites with hydrophobic domains Haupt, 1998 , has been shown to enhance binding and Ž . achieve selective recognition in aqueous solutions. Biomimetic imprinted networks were first used as carriers for drug delivery see also Figure 12 by Peppas and cowork- Ž . ers Byrne et al., 2002 . On the forefront of controlled drug Ž . Figure 10. Hydrogels may expand or contract due to pH or temperature changes. Drug diffusion may be facilitated or impeded depending on the molecular radius of the drug r with respect to the mesh size of the s polymeric gel carrier. AIChE Journal December 2003 Vol. 49, No. 12 2999�