正在加载图片...
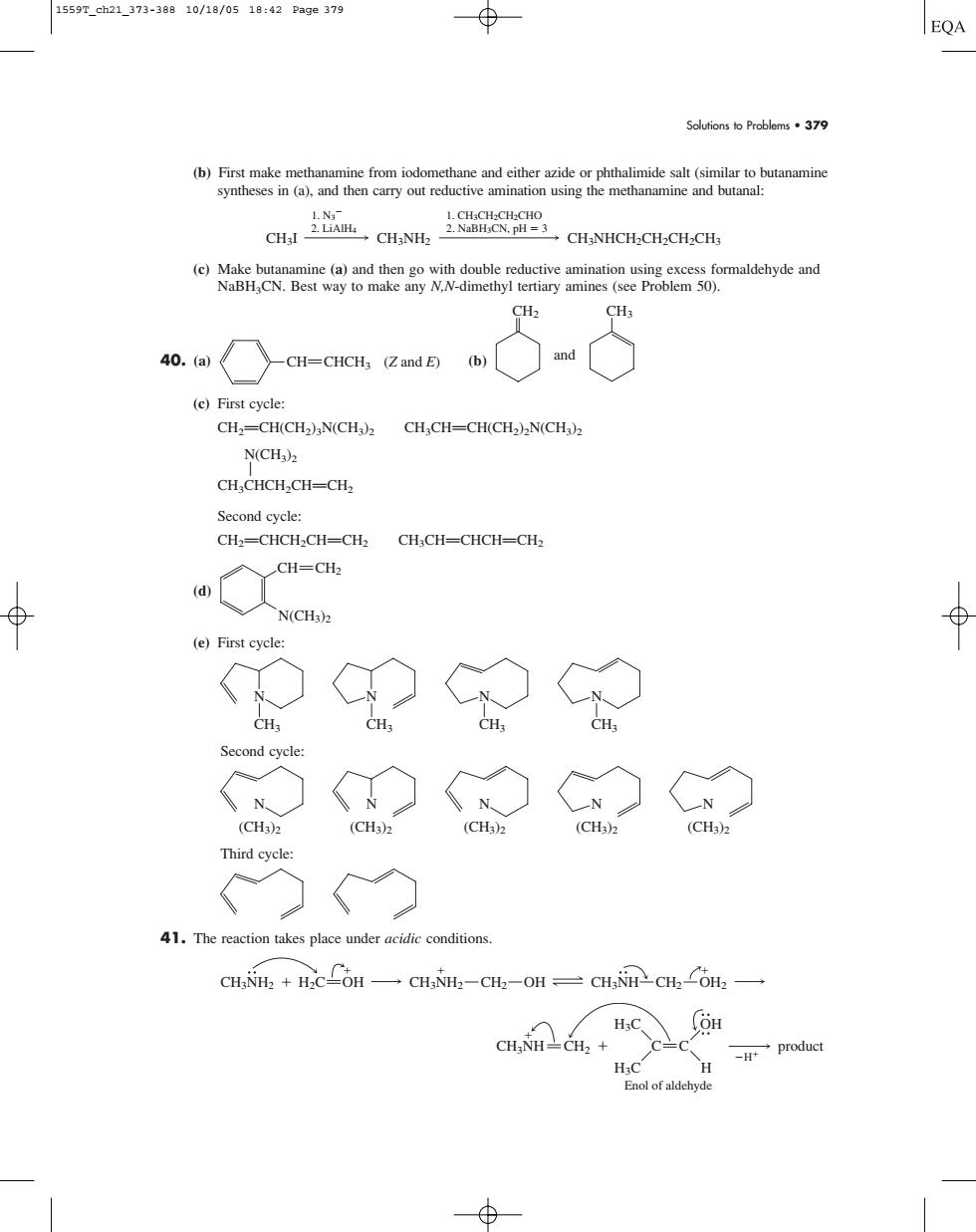
1559T_ch21_373-38810/18/0518:42Pa9e379 ⊕ EQA Solutions to Problems.379 (b)First make methar thalimide sat(similar to CHs (e)First cycle: CH2=CH(CH2)N(CH)2CH,CH-CH(CH2)2N(CH): N(CH)z CH CHCH-CH-CH Second cvclez CH2=CHCH:CH-CH CH,CH-CHCH-CH2 CH=CH, (e)First cycl O CH Second eycle ◇C◇ (CH3)2 (CH3) CH1 (CH3) Third 41.The reaction takes place under acidic conditions. CH.NH.+.COH CH.NH-CH-OH CH.+ product Enol of aldehyd Solutions to Problems • 379 (b) First make methanamine from iodomethane and either azide or phthalimide salt (similar to butanamine syntheses in (a), and then carry out reductive amination using the methanamine and butanal: (c) Make butanamine (a) and then go with double reductive amination using excess formaldehyde and NaBH3CN. Best way to make any N,N-dimethyl tertiary amines (see Problem 50). 40. (a) (b) (c) First cycle: CH2PCH(CH2)3N(CH3)2 CH3CHPCH(CH2)2N(CH3)2 N(CH3)2 A CH3CHCH2CHPCH2 Second cycle: CH2PCHCH2CHPCH2 CH3CHPCHCHPCH2 (d) (e) First cycle: Second cycle: Third cycle: 41. The reaction takes place under acidic conditions. CH3NH CH2 H3C H3C OH H C C product H Enol of aldehyde CH3NH2 CH3NH2 CH2 OH CH3NH CH2 OH2 H2C OH (CH3)2 N N N N (CH3)2 (CH3)2 (CH3)2 N (CH3)2 CH3 N CH3 N CH3 N CH3 N N(CH3)2 CH CH2 CH2 CH3 and CH ( CHCH Z and E) 3 CH3I CH3NHCH2CH2CH2CH3 1. CH3CH2CH2CHO 2. NaBH3CN, pH 3 1. N3 2. LiAlH4 CH3NH2 1559T_ch21_373-388 10/18/05 18:42 Page 379�