正在加载图片...
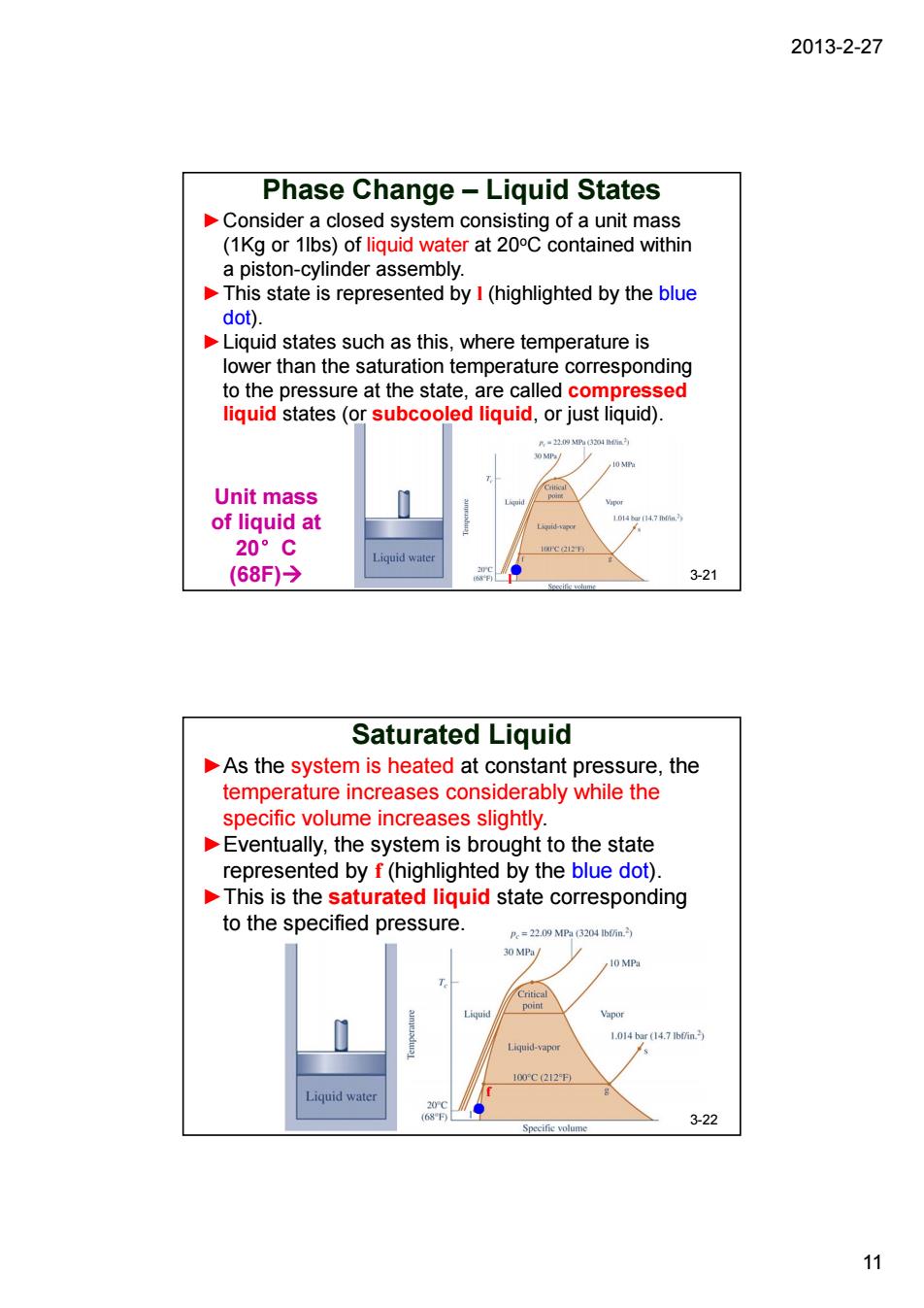
2013-2-27 Phase Change-Liquid States Consider a closed system consisting of a unit mass (1Kg or 1lbs)of liquid water at 20C contained within a piston-cvlinder assembly This state is represented by I(highlighted by the blue dot). Liquid states such as this,where temperature is lower than the saturation temperature corresponding to the pressure at the state.are called compressed liquid states (or subcooled liquid.or just liquid) Unit mass of liquid at 20°C (68F)→ 3-21 Saturated Liquid As the system is heated at constant pressure,the temperature increases considerably while the specific volume increases slightly. Eventually,the system is brought to the state represented by f(highlighted by the blue dot) This is the saturated liquid state corresponding to the specified pressure =220丹MP%(3204n 0h/ O MP 0YC212 Liquid wate .22 12013-2-27 11 ●l Phase Change – Liquid States ►Consider a closed system consisting of a unit mass (1Kg or 1lbs) of liquid water at 20oC contained within a piston-cylinder assembly. ►This state is represented by l (highlighted by the blue dot). ►Liquid states such as this, where temperature is lower than the saturation temperature corresponding to the pressure at the state, are called compressed liquid states (or subcooled liquid, or just liquid). 3-21 Unit mass of liquid at 20°C (68F)Æ Saturated Liquid ● f 3-22 ►As the system is heated at constant pressure, the temperature increases considerably while the specific volume increases slightly. ►Eventually, the system is brought to the state represented by f (highlighted by the blue dot). ►This is the saturated liquid state corresponding to the specified pressure