正在加载图片...
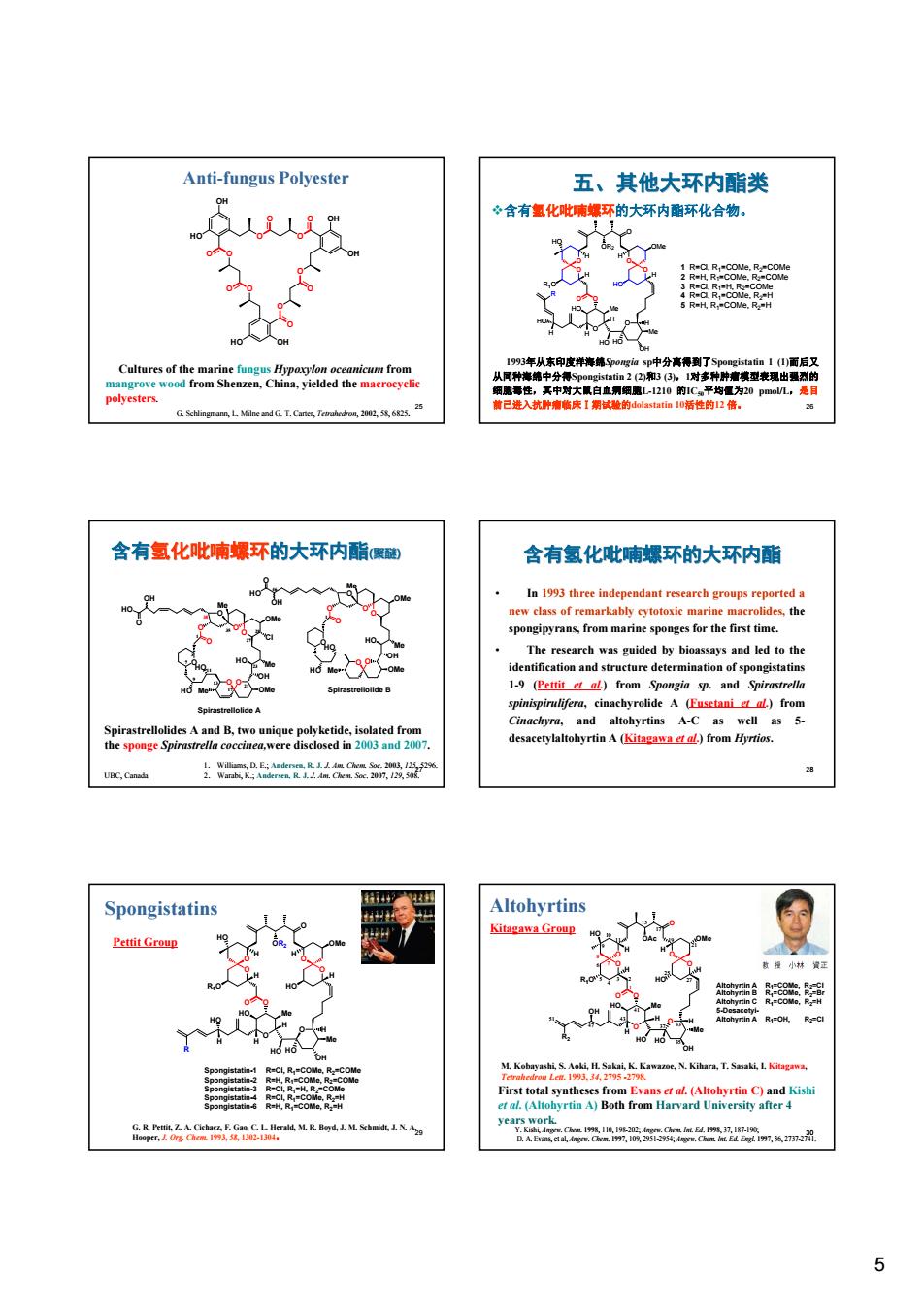
Anti-fungus Polyester 五、其他大环内酯类 心含有氢化吡哺博环的大环内酯环化合物。 含有氢化吡喃螺环的大环内酯聚壁 含有氢化吡哺螺环的大环内酯 d aL)from ns A-( Spongistatins Altohyrtins 之m 5 25 Anti-fungus Polyester Cultures of the marine fungus Hypoxylon oceanicum from mangrove wood from Shenzen, China, yielded the macrocyclic polyesters. G. Schlingmann, L. Milne and G. T. Carter, Tetrahedron, 2002, 58, 6825. 多 聚 内 酯 类 O O O O O O O O O O O O HO OH OH HO OH OH 26 五、其他大环内酯类 v含有氢化吡喃螺环的大环内酯环化合物。 O O O Me OH O O O OR2 HO O O HO Me O HO H R HO R1O HO OMe H H H HO H H H H 1 R=Cl, R1=COMe, R2=COMe 2 R=H, R1=COMe, R2=COMe 3 R=Cl, R1=H, R2=COMe 4 R=Cl, R1=COMe, R2=H 5 R=H, R1=COMe, R2=H 1993年从东印度洋海绵Spongia sp中分离得到了Spongistatin 1 (1)而后又 从同种海绵中分得Spongistatin 2 (2)和3 (3),1对多种肿瘤模型表现出强烈的 细胞毒性,其中对大鼠白血病细胞L-1210 的IC50平均值为20 pmol/L,是目 前已进入抗肿瘤临床Ⅰ期试验的dolastatin 10活性的12 倍。 27 含有氢化吡喃螺环的大环内酯(聚醚) Spirastrellolides A and B, two unique polyketide, isolated from the sponge Spirastrella coccinea,were disclosed in 2003 and 2007. 1. Williams, D. E.; Andersen, R. J. J. Am. Chem. Soc. 2003, 125, 5296. UBC, Canada 2. Warabi, K.; Andersen, R. J. J. Am. Chem. Soc. 2007, 129, 508. O O O O HO O O O HO O Me OMe OH Me Cl OMe Me O HO HO OH O O O O HO O O HO O O OH Me OMe OH Me OMe Me O HO HO 1 3 7 9 11 13 17 21 23 27 35 28 38 1 46 Spirastrellolide A Spirastrellolide B 28 • In 1993 three independant research groups reported a new class of remarkably cytotoxic marine macrolides, the spongipyrans, from marine sponges for the first time. • The research was guided by bioassays and led to the identification and structure determination of spongistatins 1-9 (Pettit et al.) from Spongia sp. and Spirastrella spinispirulifera, cinachyrolide A (Fusetani et al.) from Cinachyra, and altohyrtins A-C as well as 5- desacetylaltohyrtin A (Kitagawa et al.) from Hyrtios. 含有氢化吡喃螺环的大环内酯 29 G. R. Pettit, Z. A. Cichacz, F. Gao, C. L. Herald, M. R. Boyd, J. M. Schmidt, J. N. A. Hooper, J. Org. Chem. 1993, 58, 1302-1304。 Spongistatins Pettit Group O O O Me OH O O O OR2 HO O O HO Me O HO H R HO R1O HO OMe H H H HO H H H H Spongistatin-1 R=Cl, R1=COMe, R2=COMe Spongistatin-2 R=H, R1=COMe, R2=COMe Spongistatin-3 R=Cl, R1=H, R2=COMe Spongistatin-4 R=Cl, R1=COMe, R2=H Spongistatin-6 R=H, R1=COMe, R2=H 30 M. Kobayashi, S. Aoki, H. Sakai, K. Kawazoe, N. Kihara, T. Sasaki, I. Kitagawa, Tetrahedron Lett. 1993, 34, 2795 -2798. First total syntheses from Evans et al. (Altohyrtin C) and Kishi et al. (Altohyrtin A) Both from Harvard University after 4 years work. Y. Kishi, Angew. Chem. 1998, 110, 198-202; Angew. Chem. Int. Ed. 1998, 37, 187-190; D. A. Evans, et al, Angew. Chem. 1997, 109, 2951-2954; Angew. Chem. Int. Ed. Engl. 1997, 36, 2737-2741. Kitagawa Group O O O Me OH O O O OAc HO O O HO Me O HO H OH R1O HO OMe H H H HO H H H Altohyrtin A R1=COMe, R2=Cl Altohyrtin B R1=COMe, R2=Br Altohyrtin C R1=COMe, R2=H 5-DesacetyiAltohyrtin A R1=OH, R2=Cl R2 3 2 4 5 6 7 8 9 10 11 15 17 19 21 25 27 33 35 37 41 43 47 51 1 Altohyrtins