正在加载图片...
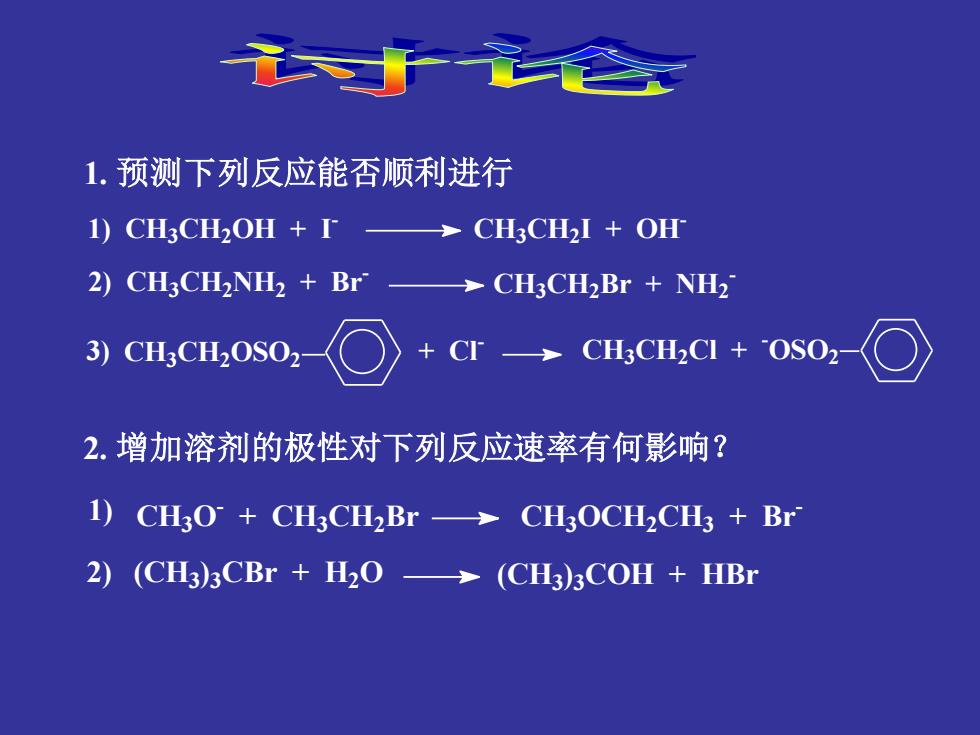
1.预测下列反应能否顺利进行 1)CH3CH2OH +I CH3CH2I+OH 2)CH3CH2NH2+Br>CH3CH2Br NH2 3)CH3CH2OSO2 →CH3CH2C+0S02 2.增加溶剂的极性对下列反应速率有何影响? 1)CH3O+CH3CH2Br->CH3OCH2CH3 Br 2)(CH3)3CBr H2O->(CH3)3COH HBr 1. 预测下列反应能否顺利进行 1) CH3CH2OH + I - CH3CH2 I + OH - 2) CH3CH2NH2 + Br - CH3CH2Br + NH2 - CH3CH2OSO2 + Cl - CH3CH2Cl + - 3) OSO2 2. 增加溶剂的极性对下列反应速率有何影响? CH3O - + CH3CH2 Br CH3OCH2CH3 + Br - (CH3) 3CBr + H2O (CH3 ) 3COH + HBr 1) 2)