正在加载图片...
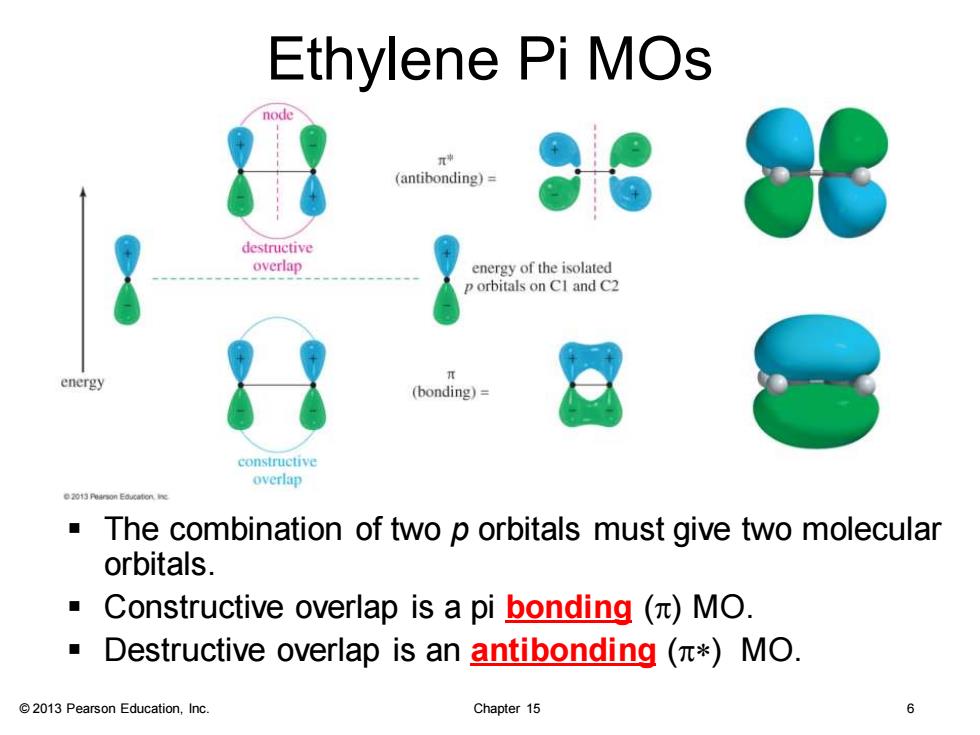
Ethylene Pi MOs node (antibonding)= destructive overlap energy of the isolated orbitals on CI and C2 energy (bonding)= constructive overlap The combination of two p orbitals must give two molecular orbitals. ■ Constructive overlap is a pi bonding()MO. Destructive overlap is an antibonding (*MO. 2013 Pearson Education,Inc. Chapter 15 © 2013 Pearson Education, Inc. Chapter 15 6 Ethylene Pi MOs ▪ The combination of two p orbitals must give two molecular orbitals. ▪ Constructive overlap is a pi bonding (p) MO. ▪ Destructive overlap is an antibonding (p*) MO