正在加载图片...
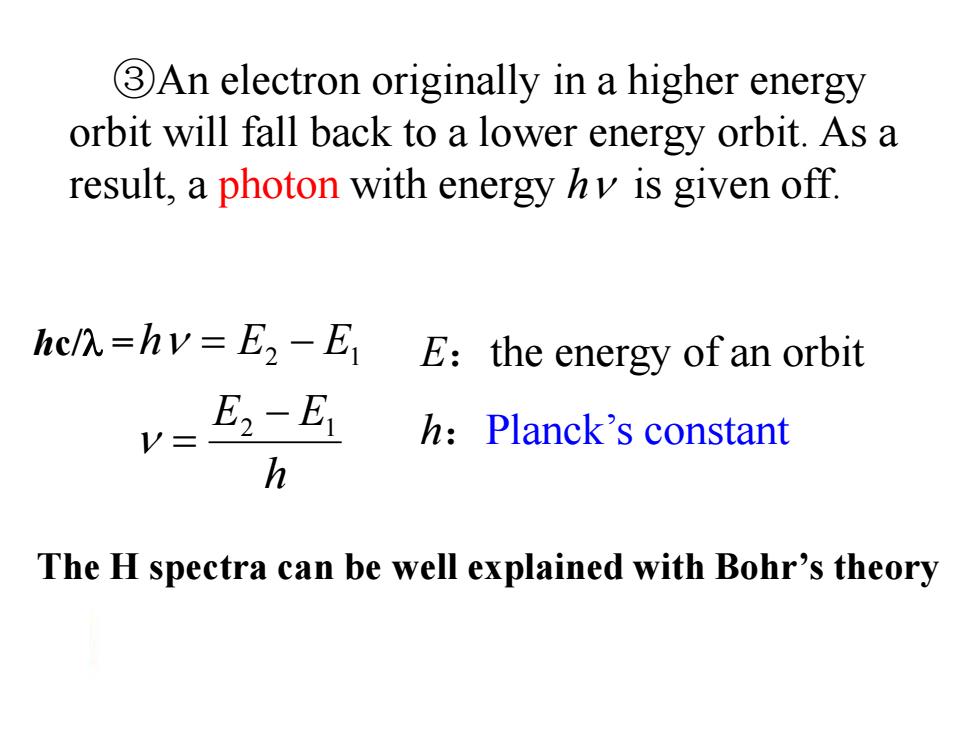
3An electron originally in a higher energy orbit will fall back to a lower energy orbit.As a result,a photon with energy hv is given off. hcl=hv=E2-E E:the energy of an orbit E2-E1 h:Planck's constant h The H spectra can be well explained with Bohr's theory ③An electron originally in a higher energy orbit will fall back to a lower energy orbit. As a result, a photon with energy h is given off. h E E h E E 2 1 2 1 E:the energy of an orbit h:Planck’s constant The H spectra can be well explained with Bohr’s theory hc/ =