正在加载图片...
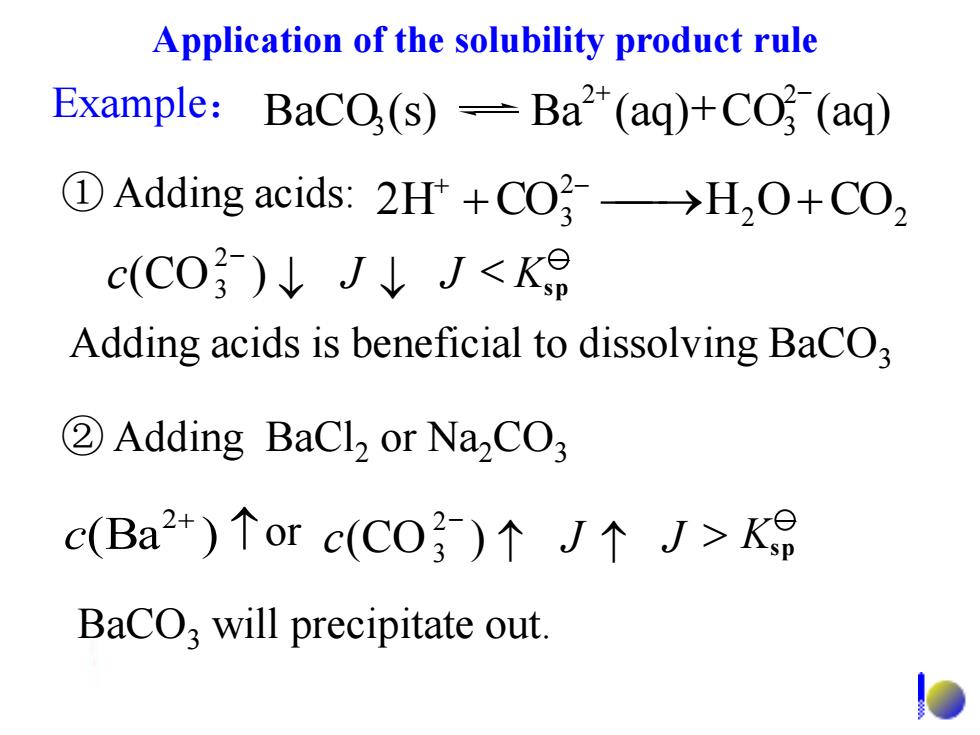
Application of the solubility product rule Example:BaCO(s)-Ba2(aq)+CO (aq) ①Adding acids:2Ht+CO}→H,O+CO, c(CO3)↓J↓J<K Adding acids is beneficial to dissolving BaCO 2Adding BaCl2 or Na2CO3 c(Ba2+)个orc(C0})个J个J>K BaCO,will precipitate outExample: 2 2 2 2H +CO3 H O+CO + - BaCO3 will precipitate out. ② Adding BaCl2 or Na2CO3 ① Adding acids: BaCO (s) Ba (aq) CO (aq) 2 3 2 3 + - + Adding acids is beneficial to dissolving BaCO3 2 3 c(CO ) J J < - Ksp or 2 3 c(CO ) J J > - + (Ba ) 2 c Ksp Application of the solubility product rule