正在加载图片...
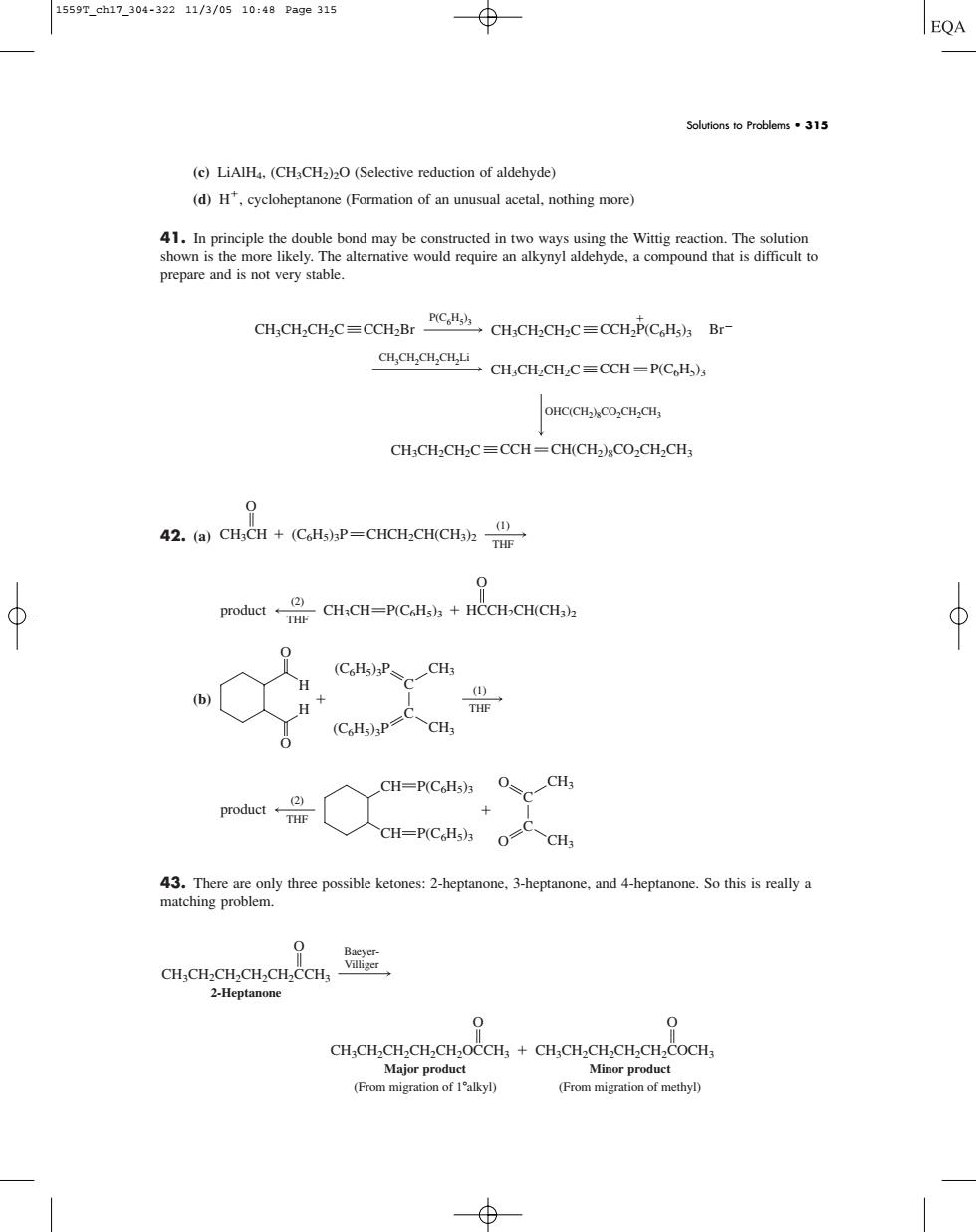
1559r.ch17304-32211/3/0510:48Page315 Solutions to Problems315 (c)LiAH.(CH,CH2)2(Selective reduction of aldehyde) (d)H',cycloheptanone (Formation of an unusual acetal,nothing more) 41.In principle the double bond may be constructed in two ways using the Wittig reaction.The solution prepare and CH.CH.CH.CCCH.BrCH,CH-CHC= CH,CH.CHC=CCH-PCH OHC..CH.CH CH:CH2CH2C=CCH-CH(CH2CO2CH2CHs 42.(a)CHCH+(CoHs)sP=CHCH:CH(CHs)z 0 poduet CHCH-PCH)+HCCHCHCH) (b) H CH=P(CoHs)a O、CH CH-P(CH3)3 oCH GLGLOLOLOREG 2-Heptanone 0 0 CH.CH.CH.CH.CH.OCCH+CH.CH.CH.CH.CH.COCH (From migration of alkyl) (From migrtio of mcthy)(c) LiAlH4, (CH3CH2)2O (Selective reduction of aldehyde) (d) H, cycloheptanone (Formation of an unusual acetal, nothing more) 41. In principle the double bond may be constructed in two ways using the Wittig reaction. The solution shown is the more likely. The alternative would require an alkynyl aldehyde, a compound that is difficult to prepare and is not very stable. 42. (a) (b) 43. There are only three possible ketones: 2-heptanone, 3-heptanone, and 4-heptanone. So this is really a matching problem. CH3CH2CH2CH2CH2OCCH3 O Major product (From migration of 1
alkyl) CH3CH2CH2CH2CH2COCH3 O Minor product (From migration of methyl) BaeyerVilliger CH3CH2CH2CH2CH2CCH3 O 2-Heptanone (2) THF O CH3 O CH3 C C CH CH P(C6H5)3 P(C6H5)3 product (1) THF O H H O (C6H5)3P CH3 (C6H5)3P CH3 C C (2) THF product CH3CH P(C6H5)3 HCCH2CH(CH3)2 O (1) THF CH3CH (C6H5)3P CHCH2CH(CH3)2 O P(C6H5)3 OHC(CH2)8CO2CH2CH3 CH3CH2CH2CH2Li CH3CH2CH2C CCH2Br CH3CH2CH2C CCH2P(C6H5)3 Br CH3CH2CH2C CCH P(C6H5)3 CH3CH2CH2C CCH CH(CH2)8CO2CH2CH3 Solutions to Problems • 315 1559T_ch17_304-322 11/3/05 10:48 Page 315�