正在加载图片...
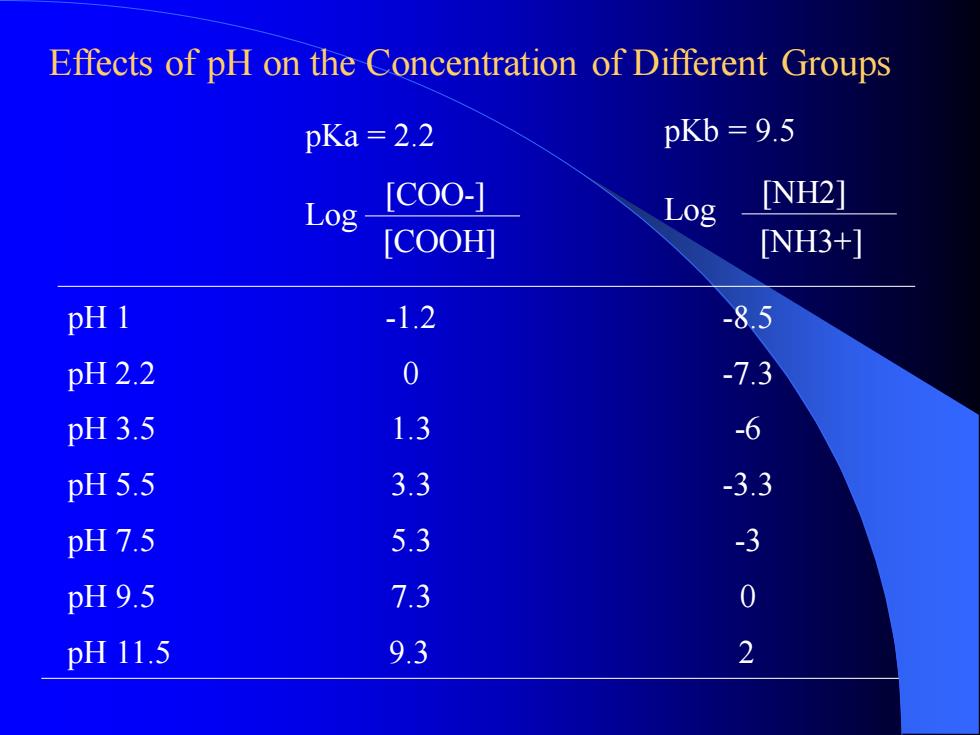
pH 1 -1.2 -8.5 pH 2.2 0 -7.3 pH 3.5 1.3 -6 pH 5.5 3.3 -3.3 pH 7.5 5.3 -3 pH 9.5 7.3 0 pKa = 2.2 Log pKb = 9.5 Log pH 11.5 9.3 2 [COOH] [COO-] [NH3+] [NH2] Effects of pH on the Concentration of Different GroupspH 1 -1.2 -8.5 pH 2.2 0 -7.3 pH 3.5 1.3 -6 pH 5.5 3.3 -3.3 pH 7.5 5.3 -3 pH 9.5 7.3 0 pKa = 2.2 Log pKb = 9.5 Log pH 11.5 9.3 2 [COOH] [COO-] [NH3+] [NH2] Effects of pH on the Concentration of Different Groups