正在加载图片...
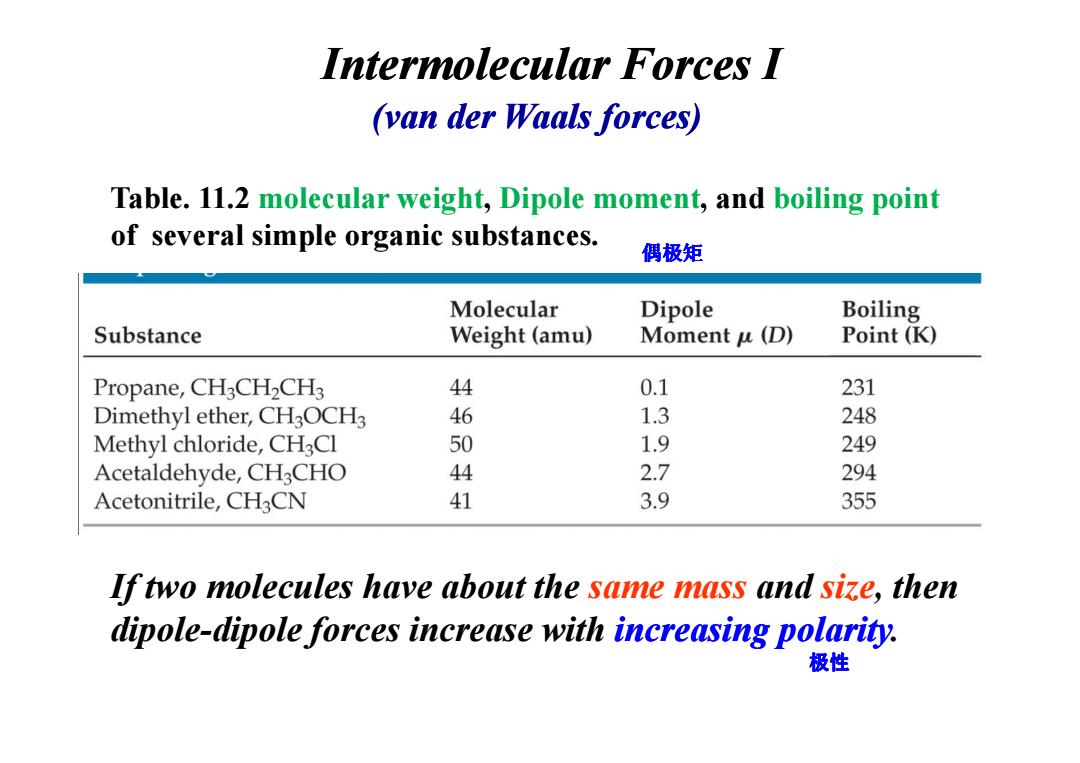
Intermolecular Forces I (van der Waals forces) Table.11.2 molecular weight,Dipole moment,and boiling point of several simple organic substances. 偶极矩 Molecular Dipole Boiling Substance Weight (amu) Moment u(D) Point(K) Propane,CH3CH2CH3 44 0.1 231 Dimethyl ether,CH3OCH3 46 1.3 248 Methyl chloride,CH3Cl 50 1.9 249 Acetaldehyde,CH3CHO 44 2.7 294 Acetonitrile,CH3CN 41 3.9 355 If two molecules have about the same mass and size,then dipole-dipole forces increase with increasing polarity. 极性Intermolecular Forces I (van der Waals forces) Table. 11.2 molecular weight, Dipole moment, and boiling point of several simple organic substances. 偶极矩 If two molecules have about the same mass and size, then dipole-dipole forces increase with increasing polarity. 极性