正在加载图片...
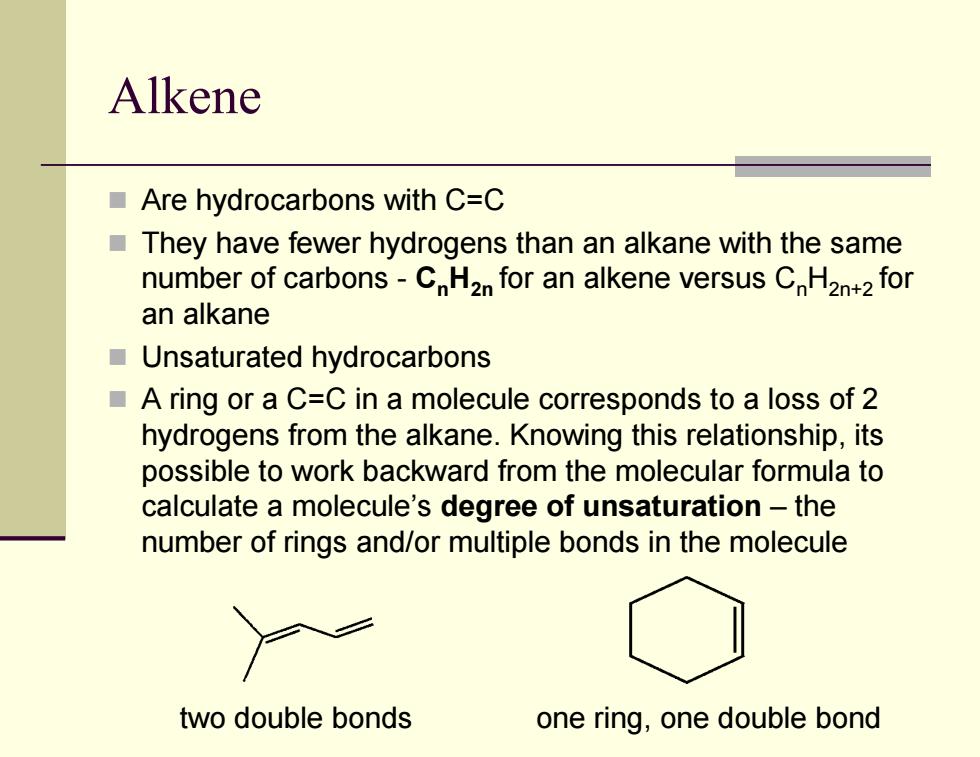
Alkene Are hydrocarbons with C=C They have fewer hydrogens than an alkane with the same number of carbons-CH2n for an alkene versus CH2n+2 for an alkane Unsaturated hydrocarbons A ring or a C=C in a molecule corresponds to a loss of 2 hydrogens from the alkane.Knowing this relationship,its possible to work backward from the molecular formula to calculate a molecule's degree of unsaturation-the number of rings and/or multiple bonds in the molecule two double bonds one ring,one double bondAlkene Are hydrocarbons with C=C They have fewer hydrogens than an alkane with the same number of carbons - CnH2n for an alkene versus CnH2n+2 for an alkane Unsaturated hydrocarbons A ring or a C=C in a molecule corresponds to a loss of 2 hydrogens from the alkane. Knowing this relationship, its possible to work backward from the molecular formula to calculate a molecule’s degree of unsaturation – the number of rings and/or multiple bonds in the molecule two double bonds one ring, one double bond