正在加载图片...
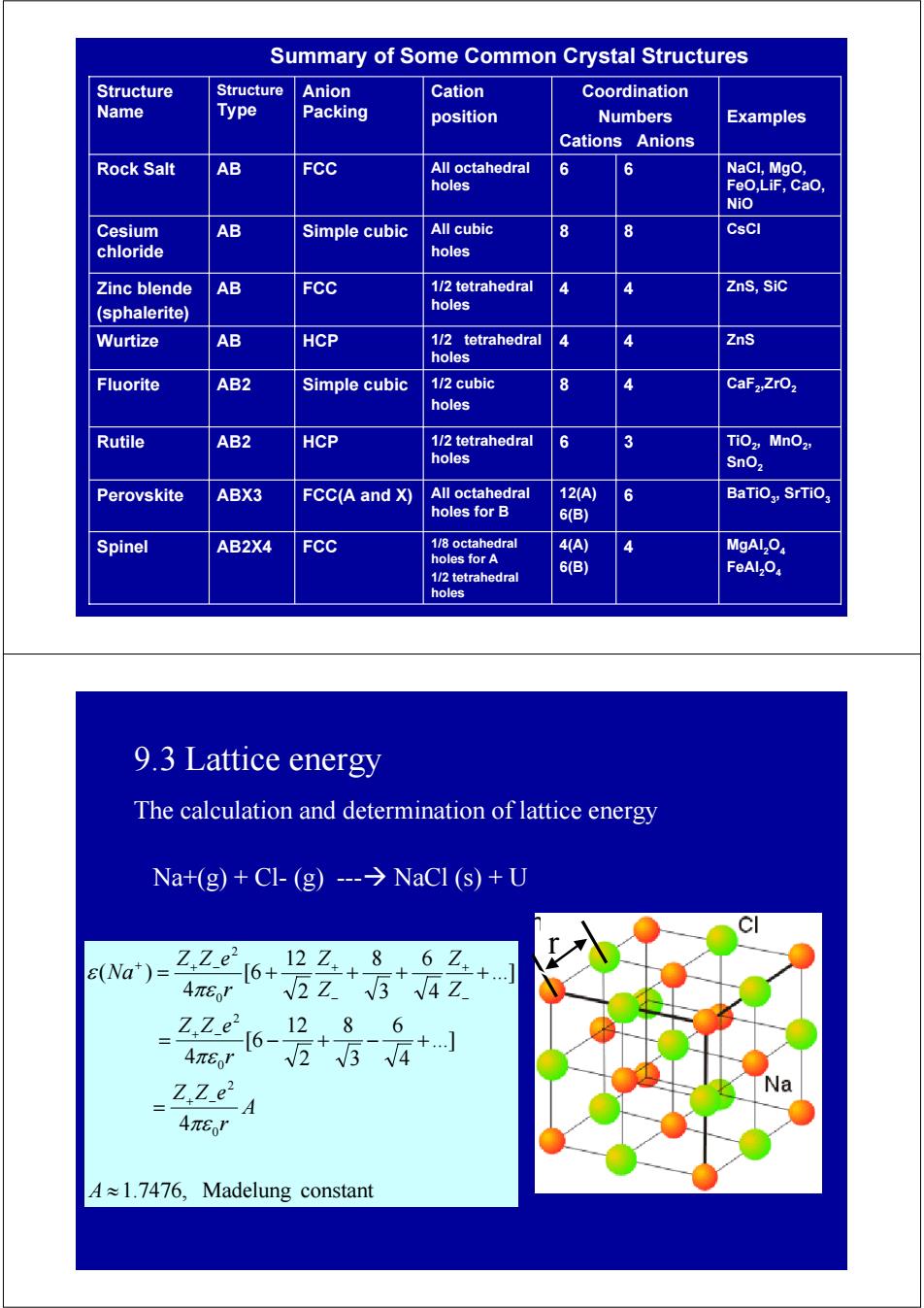
Summary of Some Common Crystal Structures Structure Structure Anion Cation Coordination Name Type Packing position Numbers Examples Cations Anions Rock Salt AB FCC All octahedral 6 6 NaCl,MgO, holes FeO,LiF,CaO, NiO Cesium AB Simple cubic All cubic 8 8 CsCI chloride holes Zinc blende AB FCC 1/2 tetrahedral 4 ZnS,Sic (sphalerite) holes Wurtize AB HCP 1/2 tetrahedral 4 ZnS holes Fluorite AB2 Simple cubic 1/2 cubic 8 CaF2:ZrO2 holes Rutile AB2 HCP 1/2 tetrahedral 6 3 TiO2,MnO2, holes SnO2 Perovskite ABX3 FCC(A and X) All octahedral 12(A) 6 BaTiO3,SrTiO, holes for B 6(B) Spinel AB2X4 FCC 1/8 octahedral 4A) MgALO holes for A 6(B)】 1/2 tetrahedral FeAl2O4 holes 9.3 Lattice energy The calculation and determination of lattice energy Na+(g)+Cl-(g)--->NaCl (s)+U (Na)= .Ze6+l2Z+8+62+J 一6+ 4πE 2 Z34 Z Z.Ze2 12.86 -「6- 4πEo' +... V25√4 Z.ZeA Na 4πer A≈1.7476,Madelung constantCaF2 8 4 ,ZrO2 1/2 cubic holes Fluorite AB2 Simple cubic FCC FCC(A and X) HCP HCP FCC Simple cubic FCC Anion Packing 1/2 tetrahedral 4 4 ZnS, SiC holes Zinc blende AB (sphalerite) BaTiO3 6 , SrTiO3 12(A) 6(B) All octahedral holes for B Perovskite ABX3 MgAl2O4 FeAl2O4 4(A) 4 6(B) 1/8 octahedral holes for A 1/2 tetrahedral holes Spinel AB2X4 TiO2, MnO2, SnO2 1/2 tetrahedral 6 3 holes Rutile AB2 1/2 tetrahedral 4 4 ZnS holes Wurtize AB All cubic 8 8 CsCl holes Cesium AB chloride NaCl, MgO, FeO,LiF, CaO, NiO All octahedral 6 6 holes Rock Salt AB Examples Coordination Numbers Cations Anions Cation position Structure Type Structure Name Summary of Some Common Crystal Structures 9.3 Lattice energy The calculation and determination of lattice energy Na+(g) + Cl- (g) ---Æ NaCl (s) + U 1.7476, Madelung constant 4 ...] 4 6 3 8 2 12 [6 4 ...] 4 6 3 8 2 12 [6 4 ( ) 0 2 0 2 0 2 ≈ = = − + − + = + + + + + − + − − + − + + − + A A r Z Z e r Z Z e Z Z Z Z r Z Z e Na πε πε πε ε r r