正在加载图片...
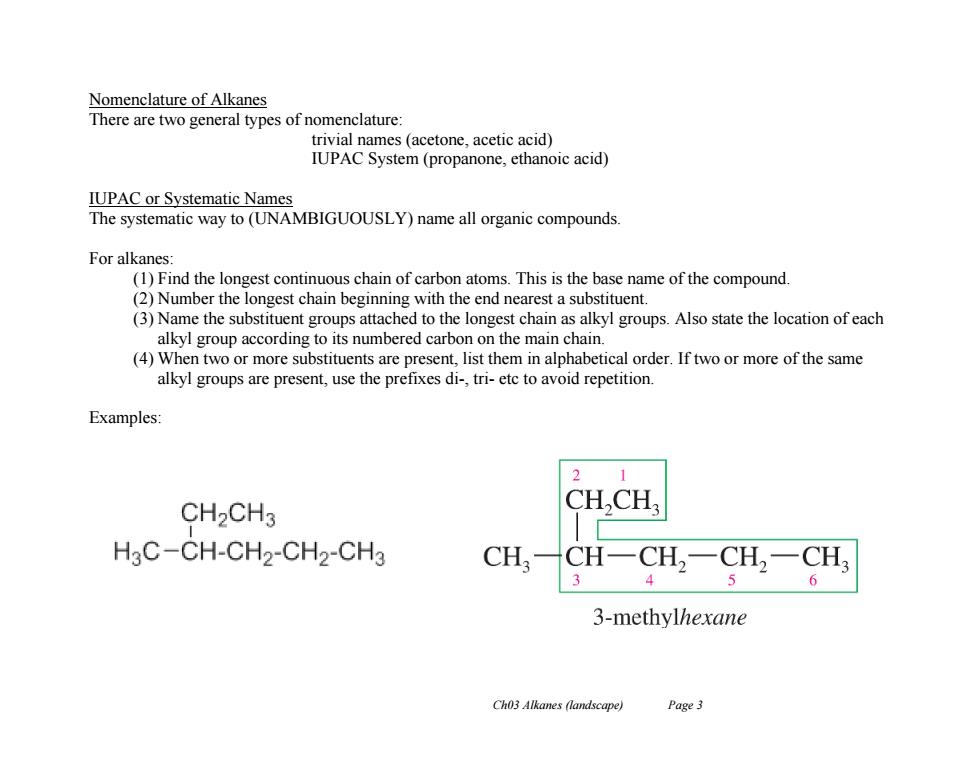
Nomenclature of Alkanes There are two general types of nomenclature trivial names (acetone,acetic acid) IUPAC System(propanone,ethanoic acid) IUPAC or Systematic Names The systematic way to(UNAMBIGUOUSLY)name all organic compounds. For alkanes: (1)Find the longest continuous chain of carbon atoms.This is the base name of the compound. (2)Number the longest chain beginning with the end nearest a substituent. (3)Name the substituent groups attached to the longest chain as alkyl groups.Also state the location of each alkyl group according to its numbered carbon on the main chain. (4)When two or more substituents are present,list them in alphabetical order.If two or more of the same alkyl groups are present,use the prefixes di-,tri-etc to avoid repetition. Examples: 1 CH2CH3 CH,CH H3C-CH-CH2-CH2-CH3 CH CH-CH2一CH2一CH3 3 4 6 3-methylhexane Ch03 Alkanes (landscape) Page 3 Ch03 Alkanes (landscape) Page 3 Nomenclature of Alkanes There are two general types of nomenclature: trivial names (acetone, acetic acid) IUPAC System (propanone, ethanoic acid) IUPAC or Systematic Names The systematic way to (UNAMBIGUOUSLY) name all organic compounds. For alkanes: (1) Find the longest continuous chain of carbon atoms. This is the base name of the compound. (2) Number the longest chain beginning with the end nearest a substituent. (3) Name the substituent groups attached to the longest chain as alkyl groups. Also state the location of each alkyl group according to its numbered carbon on the main chain. (4) When two or more substituents are present, list them in alphabetical order. If two or more of the same alkyl groups are present, use the prefixes di-, tri- etc to avoid repetition. Examples: