正在加载图片...
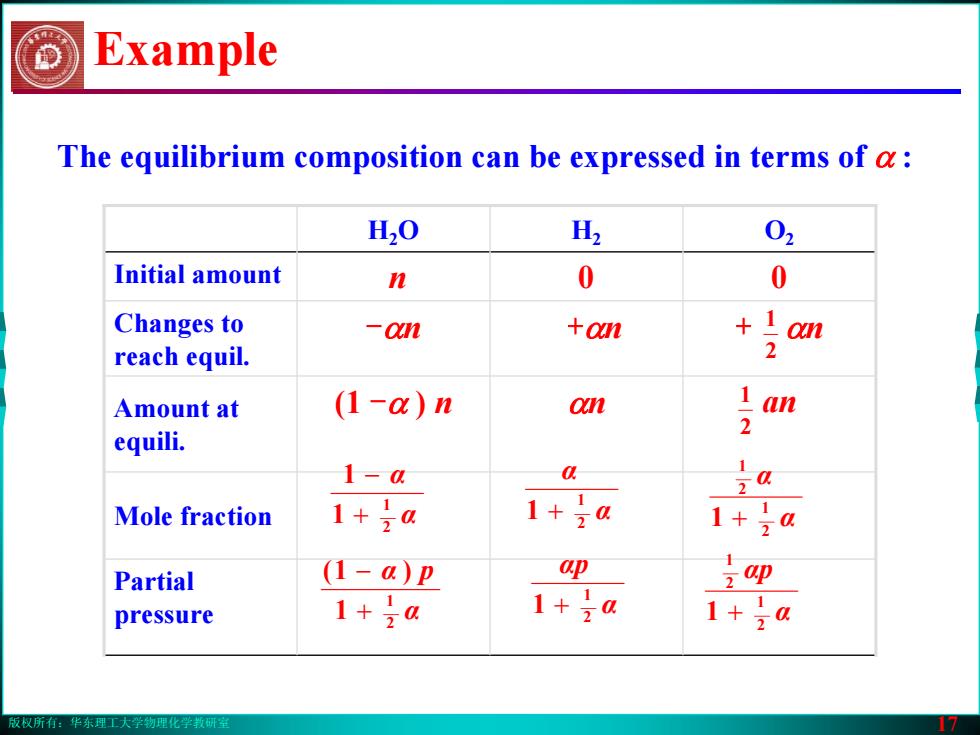
版权所有:华东理工大学物理化学教研室 17 The equilibrium composition can be expressed in terms of α : H2O H2 O2 Initial amount n 0 0 Changes to reach equil. -αn +αn + αn Amount at equili. (1 -α ) n αn an Mole fraction Partial pressure Example α α 2 1 1 1 + − α α 2 1 1 + α α 2 1 2 1 1 + α α p 2 1 1 )1( + − α αp 2 1 1 + α αp 2 1 2 1 1 + 2 1 2 1版权所有:华东理工大学物理化学教研室 17 The equilibrium composition can be expressed in terms of α : H2O H2 O2 Initial amount n 0 0 Changes to reach equil. -αn +αn + αn Amount at equili. (1 -α ) n αn an Mole fraction Partial pressure Example α α 2 1 1 1 + − α α 2 1 1 + α α 2 1 2 1 1 + α α p 2 1 1 )1( + − α αp 2 1 1 + α αp 2 1 2 1 1 + 2 1 2 1