正在加载图片...
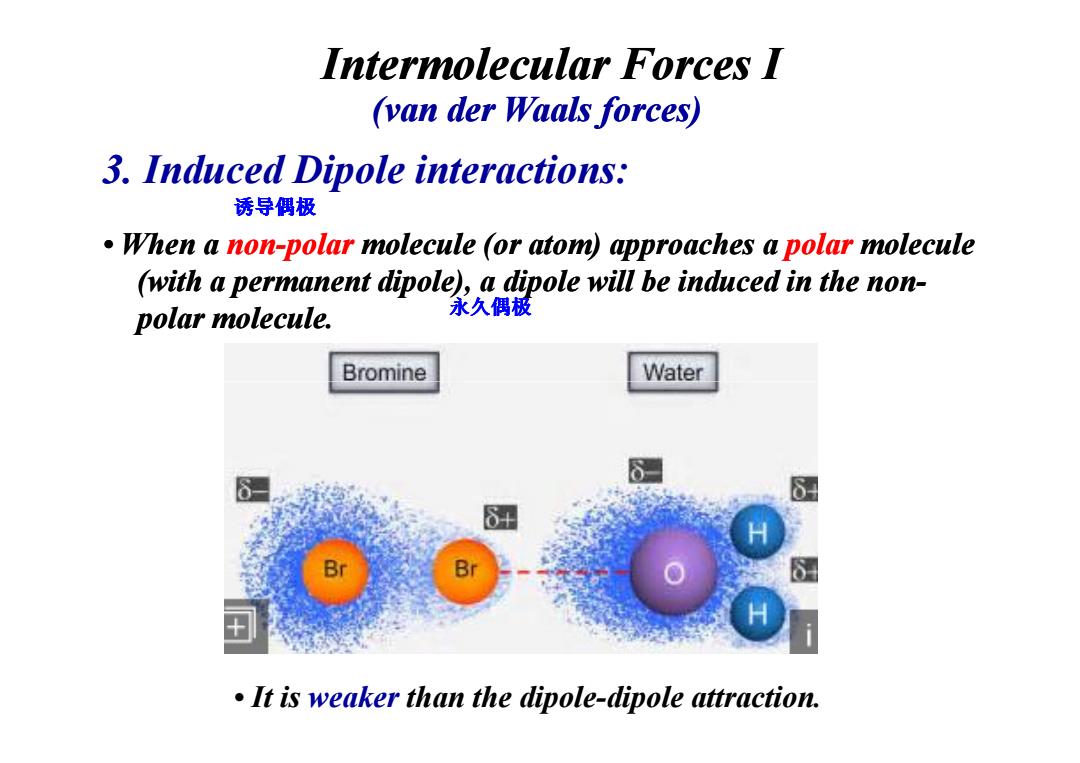
Intermolecular Forces I (van der Waals forces) 3.Induced Dipole interactions: 诱导偶极 When a non-polar molecule (or atom)approaches a polar molecule (with a permanent dipole),a dipole will be induced in the non- polar molecule. 永久偶极 Bromine Water B It is weaker than the dipole-dipole attraction.• When a non-polar molecule (or atom) approaches a polar molecule (with a permanent dipole), a dipole will be induced in the non (with a permanent dipole), a dipole will be induced in the nonpolar molecule. 3. Induced Dipole interactions: Intermolecular Forces I (van der Waals forces) Waals forces) 诱导偶极 永久偶极 • It is weaker than the dipole-dipole attraction