正在加载图片...
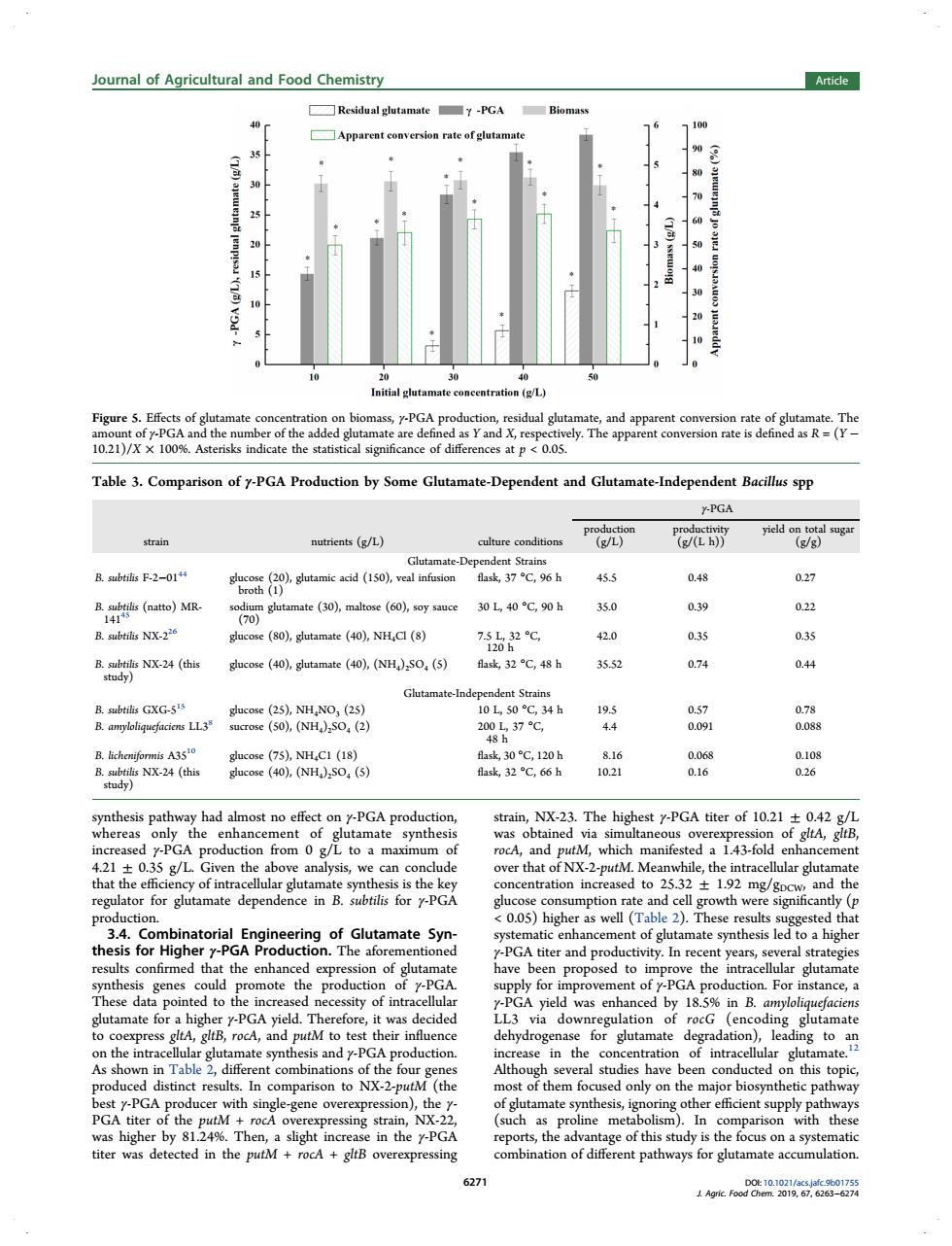
Journal of Agricultural and Food Chemistry 7-PGA Apparent conversion rate of glu 90 ration (g/L d the e add 021)/x y-PGA of th 200 at p Table 3.Comparison of-PGA Production by Some Glutamate-Dependent and Glutamate-Independent Bacills spp .PGA (L) ate-Dep dent Stra 37℃,96b 45 a48 mate (30),matose ()y 30L40℃,90h 350 02 R subtils NX.220 e (80).glutamate (40).NHCl (8) 7℃ 42.0 035 0.35 RN24(仙 glucose (40),glutamate (40),(NH).SO.(5) flask,32 C,48 h 35.5 074 04 B.subtils GXG-55 10L,50℃,34h 195 20037℃ 4.4 0) 18 k32C,66h 8 thesis n y had almost no effec -PGA produe NX-23.The hig ony the amate synthesi obt ous ov tA nalysis.we can co hat c fNX-7 while. as well (T 2. este sis for Highe PGA titer and productivity.In rec ars. al stra the emote the for eato GA 11 was enhance by 18 in B. their t the fou ease studies have this distinct result ompa NX-Z-pi (th only on path of the sing stra with thes 124 6271synthesis pathway had almost no effect on γ-PGA production, whereas only the enhancement of glutamate synthesis increased γ-PGA production from 0 g/L to a maximum of 4.21 ± 0.35 g/L. Given the above analysis, we can conclude that the efficiency of intracellular glutamate synthesis is the key regulator for glutamate dependence in B. subtilis for γ-PGA production. 3.4. Combinatorial Engineering of Glutamate Synthesis for Higher γ-PGA Production. The aforementioned results confirmed that the enhanced expression of glutamate synthesis genes could promote the production of γ-PGA. These data pointed to the increased necessity of intracellular glutamate for a higher γ-PGA yield. Therefore, it was decided to coexpress gltA, gltB, rocA, and putM to test their influence on the intracellular glutamate synthesis and γ-PGA production. As shown in Table 2, different combinations of the four genes produced distinct results. In comparison to NX-2-putM (the best γ-PGA producer with single-gene overexpression), the γ- PGA titer of the putM + rocA overexpressing strain, NX-22, was higher by 81.24%. Then, a slight increase in the γ-PGA titer was detected in the putM + rocA + gltB overexpressing strain, NX-23. The highest γ-PGA titer of 10.21 ± 0.42 g/L was obtained via simultaneous overexpression of gltA, gltB, rocA, and putM, which manifested a 1.43-fold enhancement over that of NX-2-putM. Meanwhile, the intracellular glutamate concentration increased to 25.32 ± 1.92 mg/gDCW, and the glucose consumption rate and cell growth were significantly (p < 0.05) higher as well (Table 2). These results suggested that systematic enhancement of glutamate synthesis led to a higher γ-PGA titer and productivity. In recent years, several strategies have been proposed to improve the intracellular glutamate supply for improvement of γ-PGA production. For instance, a γ-PGA yield was enhanced by 18.5% in B. amyloliquefaciens LL3 via downregulation of rocG (encoding glutamate dehydrogenase for glutamate degradation), leading to an increase in the concentration of intracellular glutamate.12 Although several studies have been conducted on this topic, most of them focused only on the major biosynthetic pathway of glutamate synthesis, ignoring other efficient supply pathways (such as proline metabolism). In comparison with these reports, the advantage of this study is the focus on a systematic combination of different pathways for glutamate accumulation. Figure 5. Effects of glutamate concentration on biomass, γ-PGA production, residual glutamate, and apparent conversion rate of glutamate. The amount of γ-PGA and the number of the added glutamate are defined as Y and X, respectively. The apparent conversion rate is defined as R = (Y − 10.21)/X × 100%. Asterisks indicate the statistical significance of differences at p < 0.05. Table 3. Comparison of γ-PGA Production by Some Glutamate-Dependent and Glutamate-Independent Bacillus spp γ-PGA strain nutrients (g/L) culture conditions production (g/L) productivity (g/(L h)) yield on total sugar (g/g) Glutamate-Dependent Strains B. subtilis F-2−0144 glucose (20), glutamic acid (150), veal infusion broth (1) flask, 37 °C, 96 h 45.5 0.48 0.27 B. subtilis (natto) MR- 14145 sodium glutamate (30), maltose (60), soy sauce (70) 30 L, 40 °C, 90 h 35.0 0.39 0.22 B. subtilis NX-226 glucose (80), glutamate (40), NH4Cl (8) 7.5 L, 32 °C, 120 h 42.0 0.35 0.35 B. subtilis NX-24 (this study) glucose (40), glutamate (40), (NH4)2SO4 (5) flask, 32 °C, 48 h 35.52 0.74 0.44 Glutamate-Independent Strains B. subtilis GXG-515 glucose (25), NH4NO3 (25) 10 L, 50 °C, 34 h 19.5 0.57 0.78 B. amyloliquefaciens LL38 sucrose (50), (NH4)2SO4 (2) 200 L, 37 °C, 48 h 4.4 0.091 0.088 B. licheniformis A3510 glucose (75), NH4C1 (18) flask, 30 °C, 120 h 8.16 0.068 0.108 B. subtilis NX-24 (this study) glucose (40), (NH4)2SO4 (5) flask, 32 °C, 66 h 10.21 0.16 0.26 Journal of Agricultural and Food Chemistry Article DOI: 10.1021/acs.jafc.9b01755 J. Agric. Food Chem. 2019, 67, 6263−6274 6271