正在加载图片...
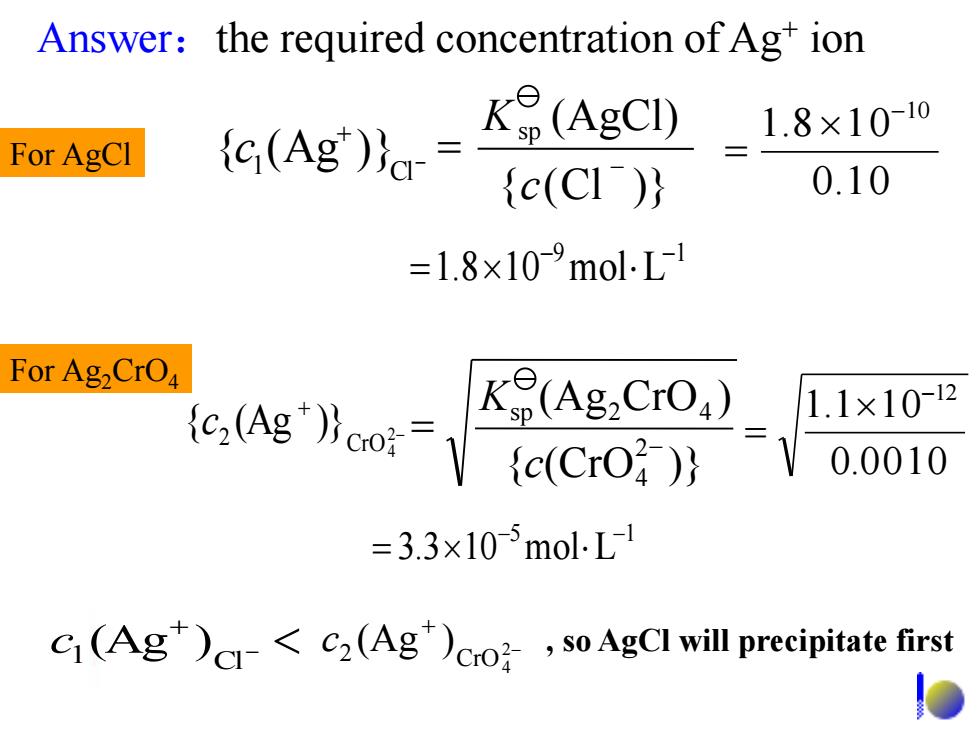
Answer:the required concentration ofAg+ion K(AgCI) 1.8×10-10 For AgCI e(Ag) (c(CI) 0.10 =1.8×10-9molL For Ag2CrO tc;(Ag")oo:= K(AgCrO) 1.1×10-2 {c(CrO 0.0010 =3.3×105molL C(Ag)<C2(Ag)cro,so AgCI will precipitate first - + 1 Cl c (Ag ) - + 2 CrO4 2 < c (Ag ) 9 1 1.8 10 mol L - - = 5 1 3.3 10 mol L - - = Answer:the required concentration of Ag+ ion - + 1 Cl {c (Ag )} 0.1 0 1.8 1 0-10 = { (Cl )} (AgCl) sp - = c K - + 2 CrO4 2 {c (Ag )} 0.0010 1.1 10-12 = { (CrO )} (Ag CrO ) 2 4 sp 2 4 - = c K , so AgCl will precipitate first For Ag2CrO4 For AgCl