正在加载图片...
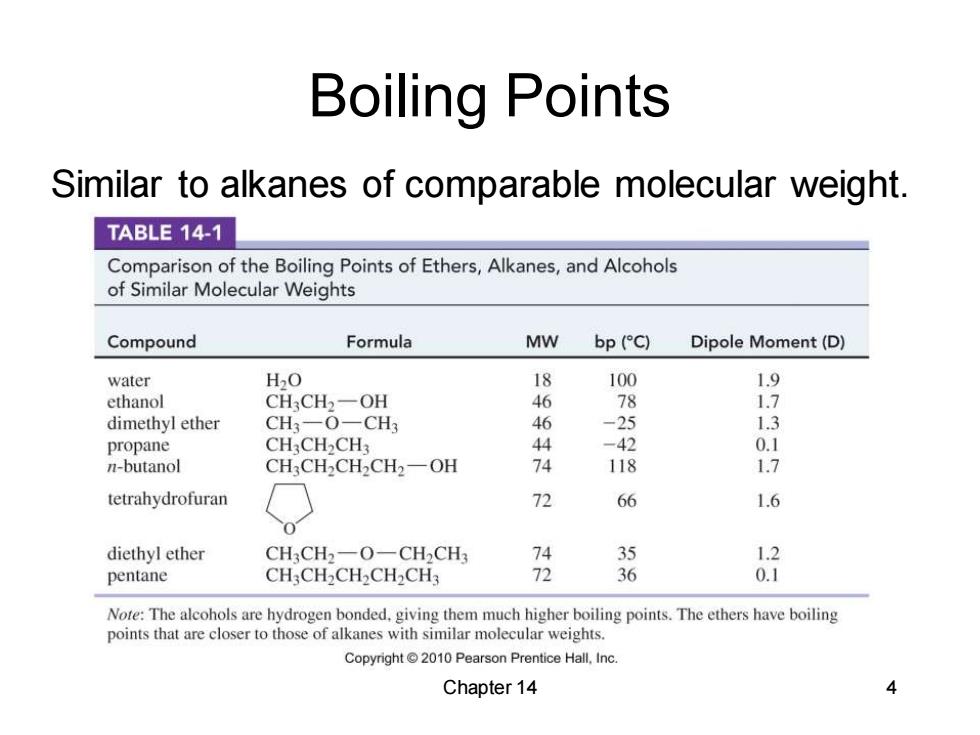
Boiling Points Similar to alkanes of comparable molecular weight. TABLE 14-1 Comparison of the Boiling Points of Ethers,Alkanes,and Alcohols of Similar Molecular Weights Compound Formula MW bp (C) Dipole Moment(D) water H2O 18 100 ethanol CH3CH2一OH 46 78 dimethyl ether CH3-O一CH3 46 -25 1.3 propane CH3CH2CH3 44 -42 n-butanol CH3CH2CH2CH2-OH 74 118 1.7 tetrahydrofuran 72 66 1.6 diethyl ether CH3CH2一O-CH2CH 35 1.2 pentane CH3CH2CH2CH2CH3 72 36 0.1 Note:The alcohols are hydrogen bonded.giving them much higher boiling points.The ethers have boiling points that are closer to those of alkanes with similar molecular weights. Copyright 2010 Pearson Prentice Hall,Inc Chapter 14 Chapter 14 4 Boiling Points Similar to alkanes of comparable molecular weight