正在加载图片...
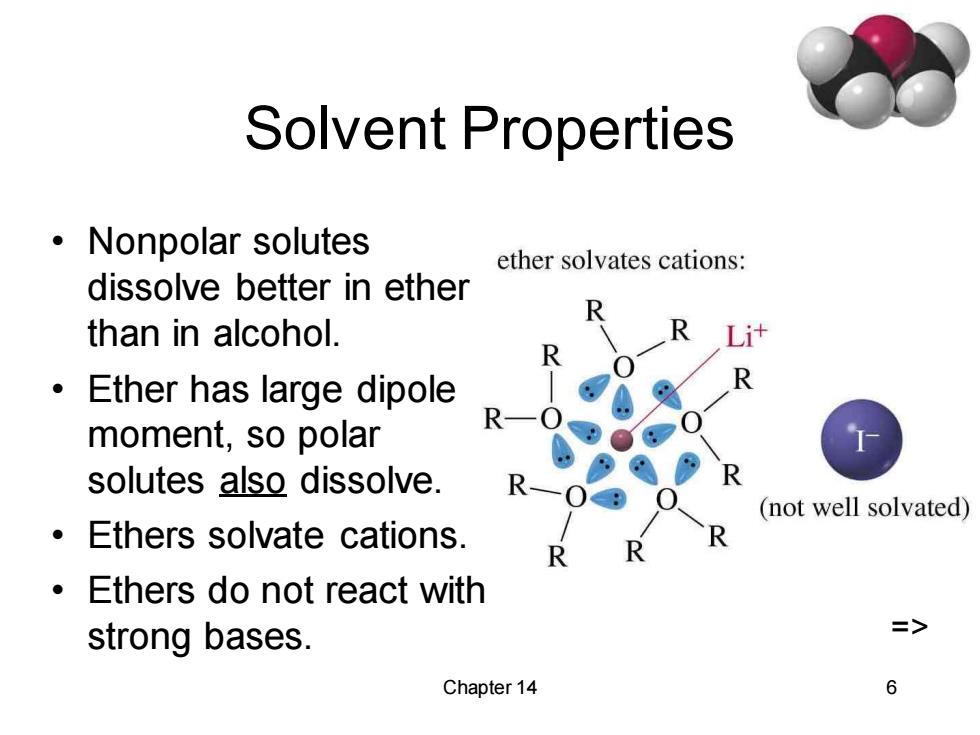
Solvent Properties ·Nonpolar solutes ether solvates cations: dissolve better in ether than in alcohol. 。 Ether has large dipole R- moment,so polar solutes also dissolve. (not well solvated) Ethers solvate cations. Ethers do not react with strong bases. => Chapter 14 6Chapter 14 6 Solvent Properties • Nonpolar solutes dissolve better in ether than in alcohol. • Ether has large dipole moment, so polar solutes also dissolve. • Ethers solvate cations. • Ethers do not react with strong bases. =>