正在加载图片...
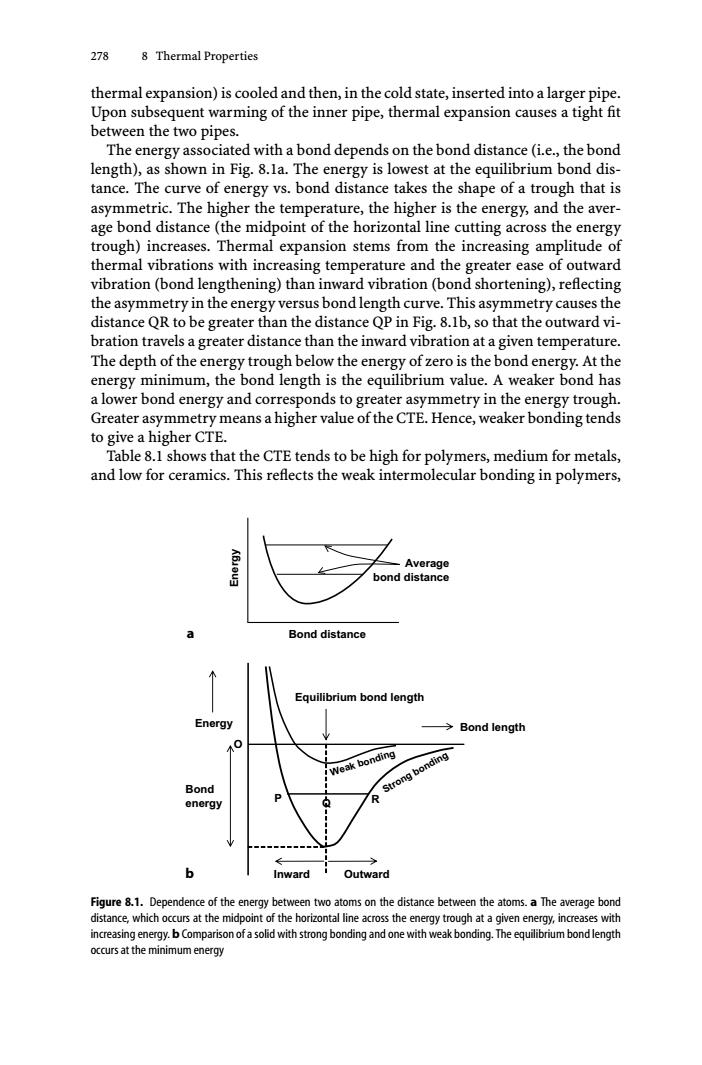
278 8 Thermal Properties thermal expansion)is cooled and then,in the cold state,inserted into a larger pipe. Upon subsequent warming of the inner pipe,thermal expansion causes a tight fit between the two pipes. The energy associated with a bond depends on the bond distance(i.e.,the bond length),as shown in Fig.8.1a.The energy is lowest at the equilibrium bond dis- tance.The curve of energy vs.bond distance takes the shape of a trough that is asymmetric.The higher the temperature,the higher is the energy,and the aver- age bond distance(the midpoint of the horizontal line cutting across the energy trough)increases.Thermal expansion stems from the increasing amplitude of thermal vibrations with increasing temperature and the greater ease of outward vibration (bond lengthening)than inward vibration(bond shortening),reflecting the asymmetry in the energy versus bond length curve.This asymmetry causes the distance QR to be greater than the distance QP in Fig.8.1b,so that the outward vi- bration travels a greater distance than the inward vibration at a given temperature. The depth of the energy trough below the energy of zero is the bond energy.At the energy minimum,the bond length is the equilibrium value.A weaker bond has a lower bond energy and corresponds to greater asymmetry in the energy trough. Greater asymmetry means a higher value of the CTE.Hence,weaker bonding tends to give a higher CTE. Table 8.1 shows that the CTE tends to be high for polymers,medium for metals, and low for ceramics.This reflects the weak intermolecular bonding in polymers, Average bond distance Bond distance Equilibrium bond length Energy →Bond length %0 Weak bonding Bond Strong bonding P R energy ← b Inward Outward Figure 8.1.Dependence of the energy between two atoms on the distance between the atoms.a The average bond distance,which occurs at the midpoint of the horizontal line across the energy trough at a given energy,increases with increasing energy.b Comparison of a solid with strong bonding and one with weak bonding.The equilibrium bond length occurs at the minimum energy278 8 Thermal Properties thermal expansion) is cooled and then, in the cold state, inserted into a larger pipe. Upon subsequent warming of the inner pipe, thermal expansion causes a tight fit between the two pipes. The energy associated with a bond depends on the bond distance (i.e., the bond length), as shown in Fig. 8.1a. The energy is lowest at the equilibrium bond distance. The curve of energy vs. bond distance takes the shape of a trough that is asymmetric. The higher the temperature, the higher is the energy, and the average bond distance (the midpoint of the horizontal line cutting across the energy trough) increases. Thermal expansion stems from the increasing amplitude of thermal vibrations with increasing temperature and the greater ease of outward vibration (bond lengthening) than inward vibration (bond shortening), reflecting the asymmetry in the energy versus bond length curve. This asymmetry causes the distance QR to be greater than the distance QP in Fig. 8.1b, so that the outward vibration travels a greater distance than the inward vibration at a given temperature. The depth of the energy trough below the energy of zero is the bond energy. At the energy minimum, the bond length is the equilibrium value. A weaker bond has a lower bond energy and corresponds to greater asymmetry in the energy trough. Greater asymmetry means a higher value of the CTE. Hence, weaker bonding tends to give a higher CTE. Table 8.1 shows that the CTE tends to be high for polymers, medium for metals, and low for ceramics. This reflects the weak intermolecular bonding in polymers, a b O Energy Bond energy Inward Bond length Outward Equilibrium bond length P Q R Weak bonding Strong bonding Energy Bond distance Average bond distance Figure 8.1. Dependence of the energy between two atoms on the distance between the atoms. a The average bond distance, which occurs at the midpoint of the horizontal line across the energy trough at a given energy, increases with increasing energy. b Comparison of a solid with strong bonding and one with weak bonding. The equilibrium bond length occurs at the minimum energy