正在加载图片...
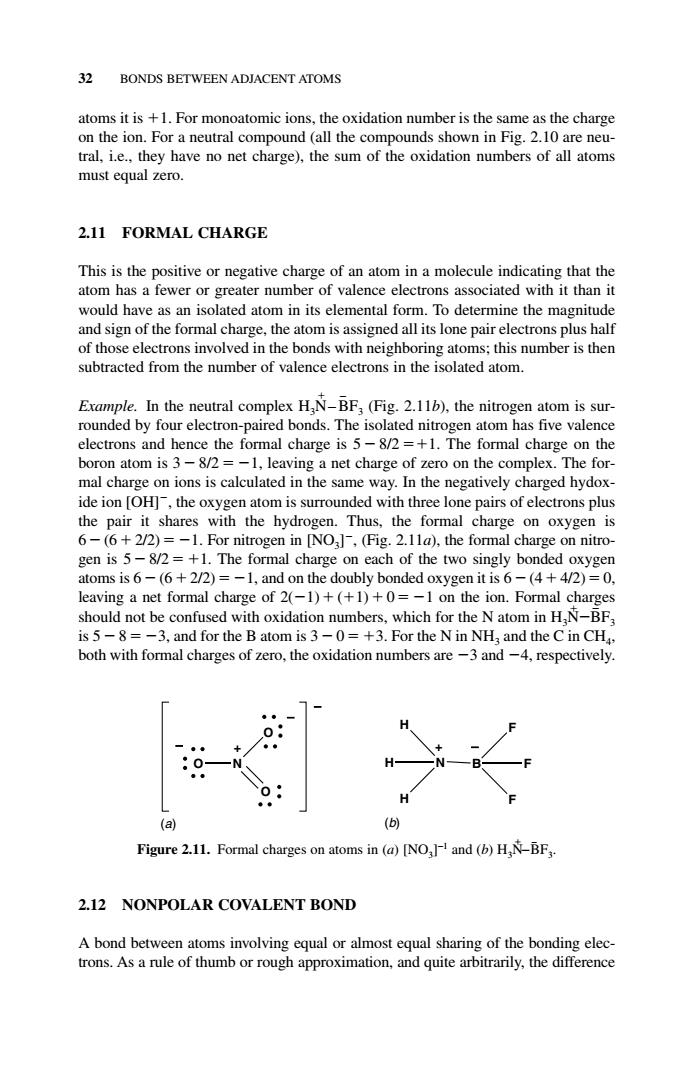
32 BONDS BETWEEN ADJACENT ATOMS atoms it is +1.For monoatomic ions,the oxidation number is the same as the charge on the ion.For a neutral compound (all the compounds shown in Fig.2.10 are neu- tral,i.e.,they have no net charge),the sum of the oxidation numbers of all atoms must equal zero. 2.11 FORMAL CHARGE This is the positive or negative charge of an atom in a molecule indicating that the atom has a fewer or greater number of valence electrons associated with it than it would have as an isolated atom in its elemental form.To determine the magnitude and sign of the formal charge,the atom is assigned all its lone pair electrons plus half of those electrons involved in the bonds with neighboring atoms;this number is then subtracted from the number of valence electrons in the isolated atom Erample.In the neutral,(Fig.2.11),the nitrogen atom is sur- rounded by four electron-paired bonds.The isolated nitrogen atom has five valence electrons and hence the formal charge is 5-8/2=+1.The formal charge on the boron atom is 3-82=-1.leaving a net charge of zero on the complex.The for mal charge on ions is calculated in the same way.In the negatively charged hydox- ide ion loHl.the oxvgen atom is surrounded with three lone pairs of electrons plus the pair it shares with the n.Thus the formal charge on oxyge n ie 6 .6+2J2)=-1.Fo N0J,(fg.2.11a the fo al ch gen is /2=+1 The form he two singl altoms is 6- 5+22 c on on the doubly bo ded oxygen (4 42)=0 eaving a net formal charge of 20-1)+(+1)+0= -1 on the ion.Formal charges should not be confused with oxidation numbers,which for the N atom in H,N-BF is 5-8=-3,and for the B atom is 3-0=+3.For the N in NH;and the C in CH. both with formal charges of zero,the oxidation numbers are-3 and-4,respectively. (a) Figure 2.11.Formal charges on atoms in (a)[NO and (b)H-BF 2.12 NONPOLAR COVALENT BOND A bond between atoms involving equal or almost equal sharing of the bonding elec- trons.As a rule of thumb or rough approximation,and quite arbitrarily,the difference atoms it is 1. For monoatomic ions, the oxidation number is the same as the charge on the ion. For a neutral compound (all the compounds shown in Fig. 2.10 are neutral, i.e., they have no net charge), the sum of the oxidation numbers of all atoms must equal zero. 2.11 FORMAL CHARGE This is the positive or negative charge of an atom in a molecule indicating that the atom has a fewer or greater number of valence electrons associated with it than it would have as an isolated atom in its elemental form. To determine the magnitude and sign of the formal charge, the atom is assigned all its lone pair electrons plus half of those electrons involved in the bonds with neighboring atoms; this number is then subtracted from the number of valence electrons in the isolated atom. Example. In the neutral complex H3N – BF3 (Fig. 2.11b), the nitrogen atom is surrounded by four electron-paired bonds. The isolated nitrogen atom has five valence electrons and hence the formal charge is 5 8/2 1. The formal charge on the boron atom is 3 8/2 1, leaving a net charge of zero on the complex. The formal charge on ions is calculated in the same way. In the negatively charged hydoxide ion [OH], the oxygen atom is surrounded with three lone pairs of electrons plus the pair it shares with the hydrogen. Thus, the formal charge on oxygen is 6 (6 2/2) 1. For nitrogen in [NO3] , (Fig. 2.11a), the formal charge on nitrogen is 5 8/2 1. The formal charge on each of the two singly bonded oxygen atoms is 6 (6 2/2) 1, and on the doubly bonded oxygen it is 6 (4 4/2) 0, leaving a net formal charge of 2(1) (1) 0 1 on the ion. Formal charges should not be confused with oxidation numbers, which for the N atom in H3N BF3 is 5 8 3, and for the B atom is 3 0 3. For the N in NH3 and the C in CH4, both with formal charges of zero, the oxidation numbers are 3 and 4, respectively. 2.12 NONPOLAR COVALENT BOND A bond between atoms involving equal or almost equal sharing of the bonding electrons. As a rule of thumb or rough approximation, and quite arbitrarily, the difference 32 BONDS BETWEEN ADJACENT ATOMS N H H H B F F F O N O O (a) (b) + − − − + − Figure 2.11. Formal charges on atoms in (a) [NO3]−1 and (b) H3N − − BF3. c02.qxd 5/17/2005 5:13 PM Page 32������������������������������