正在加载图片...
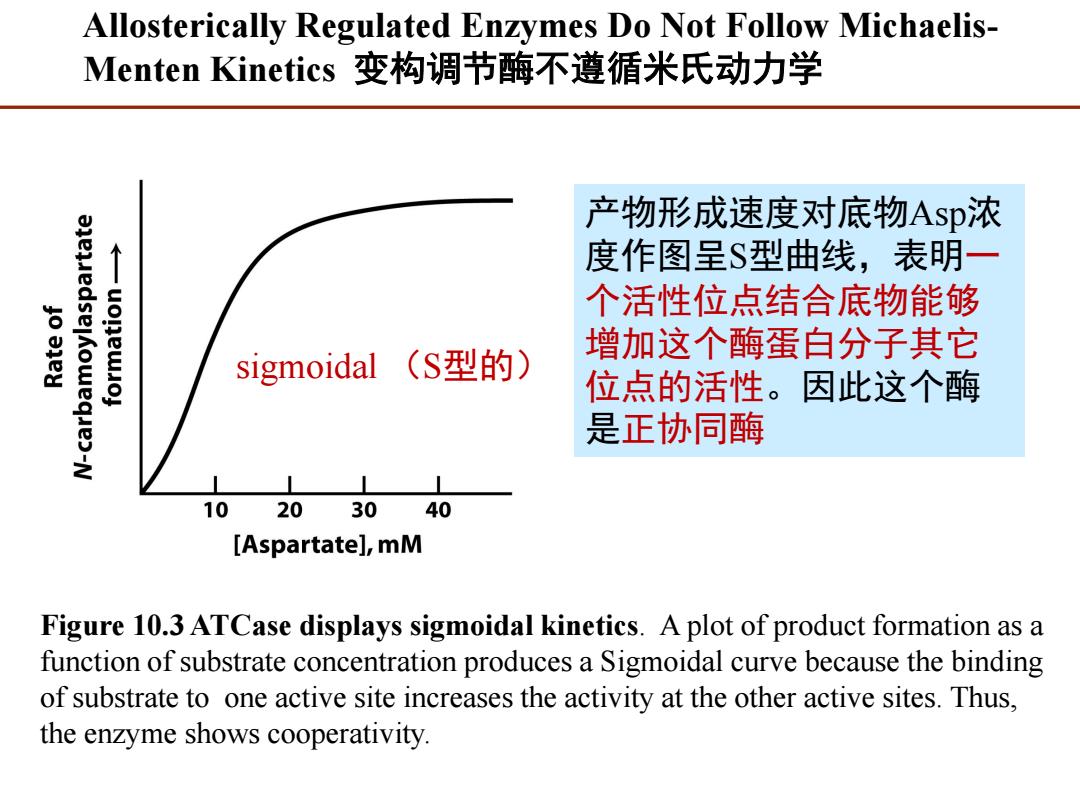
Allosterically Regulated Enzymes Do Not Follow Michaelis- Menten Kinetics变构调节酶不遵循米氏动力学 产物形成速度对底物Asp浓 度作图呈$型曲线,表明一 个活性位点结合底物能够 增加这个酶蛋白分子其它 sigmoidal (S型的) 位点的活性。因此这个酶 是正协同酶 10 20 30 40 [Aspartate],mM Figure 10.3 ATCase displays sigmoidal kinetics.A plot of product formation as a function of substrate concentration produces a Sigmoidal curve because the binding of substrate to one active site increases the activity at the other active sites.Thus, the enzyme shows cooperativity.Allosterically Regulated Enzymes Do Not Follow MichaelisMenten Kinetics 变构调节酶不遵循米氏动力学 Figure 10.3 ATCase displays sigmoidal kinetics. A plot of product formation as a function of substrate concentration produces a Sigmoidal curve because the binding of substrate to one active site increases the activity at the other active sites. Thus, the enzyme shows cooperativity. 产物形成速度对底物Asp浓 度作图呈S型曲线,表明一 个活性位点结合底物能够 增加这个酶蛋白分子其它 位点的活性。因此这个酶 是正协同酶 sigmoidal (S型的)