正在加载图片...
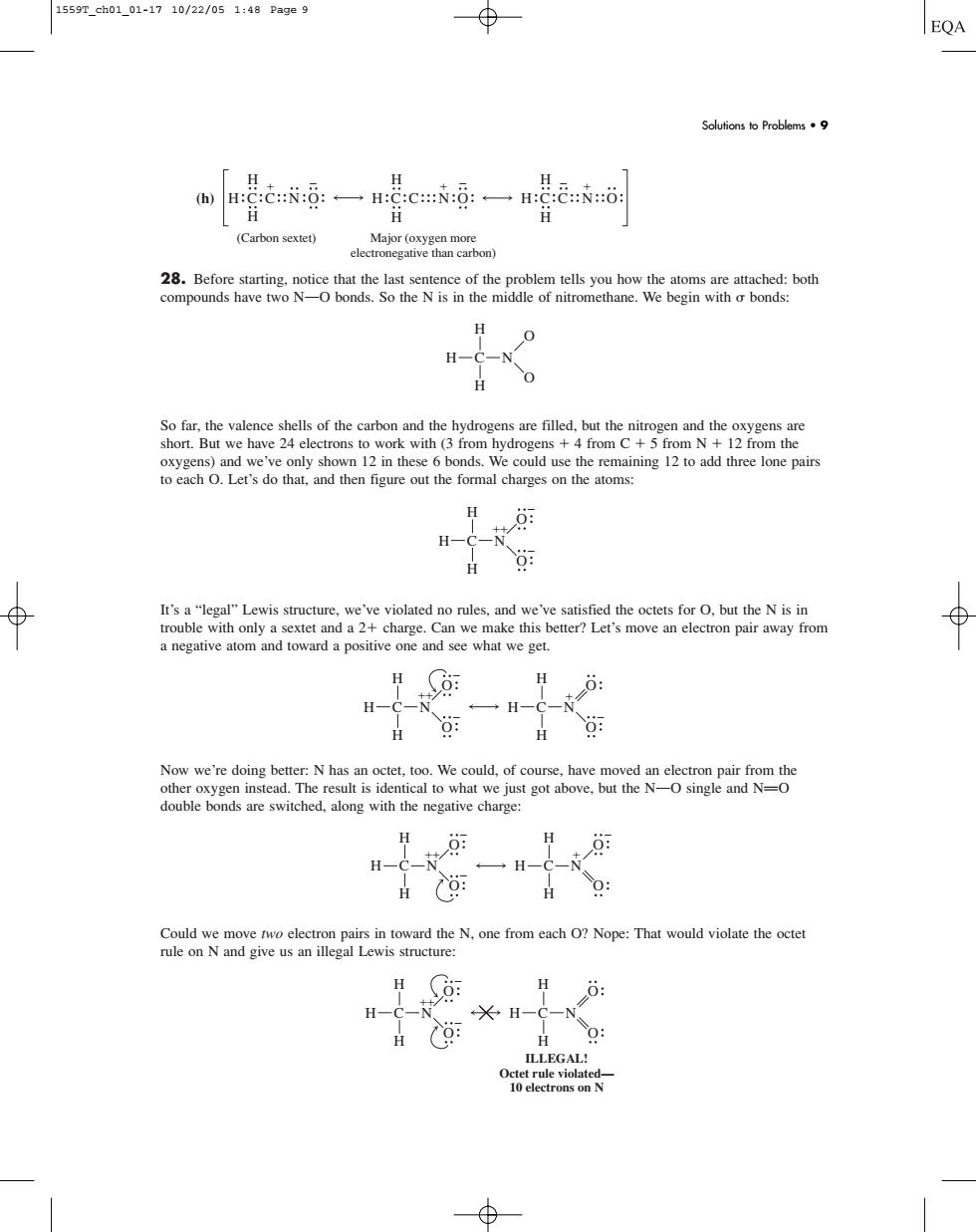
1559T_ch01_01-1710/22/051:48Page9 ⊕ EQA Solufions to Problems·9 H (Carbon sextet) H 0 H-C-N H oxygens)and we've only shown 12in these 6bonds.We could use the remaining 12 to add three lone pairs to each O.Let's do that.and then figure out the formal charges on the atoms: H H-C- It's a"legal"Lewis structure,we've violated no rules,and we've satisfied the octets for O.but the N is in trouble with only a sextet and a2+charge.Can we make this better?Let's move an elctron pair away from a negative atom and toward a positive one and see what we get. H: H-C-N No double bonds are switched,along with the negative charge: H 一H-C-N H(: : Could we move two electron pairs in toward the N.one from each O?Nope:That would violate the octet rule on N and give us an illegal Lewis structure: H o: H-C H-C H(: ILLEGAL! (h) 28. Before starting, notice that the last sentence of the problem tells you how the atoms are attached: both compounds have two NOO bonds. So the N is in the middle of nitromethane. We begin with bonds: So far, the valence shells of the carbon and the hydrogens are filled, but the nitrogen and the oxygens are short. But we have 24 electrons to work with (3 from hydrogens 4 from C 5 from N 12 from the oxygens) and we’ve only shown 12 in these 6 bonds. We could use the remaining 12 to add three lone pairs to each O. Let’s do that, and then figure out the formal charges on the atoms: It’s a “legal” Lewis structure, we’ve violated no rules, and we’ve satisfied the octets for O, but the N is in trouble with only a sextet and a 2 charge. Can we make this better? Let’s move an electron pair away from a negative atom and toward a positive one and see what we get. Now we’re doing better: N has an octet, too. We could, of course, have moved an electron pair from the other oxygen instead. The result is identical to what we just got above, but the NOO single and NPO double bonds are switched, along with the negative charge: Could we move two electron pairs in toward the N, one from each O? Nope: That would violate the octet rule on N and give us an illegal Lewis structure: H H H C N O O H H H C N O O ILLEGAL! Octet rule violated— 10 electrons on N H H H C N O O H H H C N O O H H H C N O O H H H C N O O H H H C N O O H H H C N O O H H H C C N O H H H C C N O H H H C C N O (Carbon sextet) Major (oxygen more electronegative than carbon) Solutions to Problems • 9 1559T_ch01_01-17 10/22/05 1:48 Page 9�������������