正在加载图片...
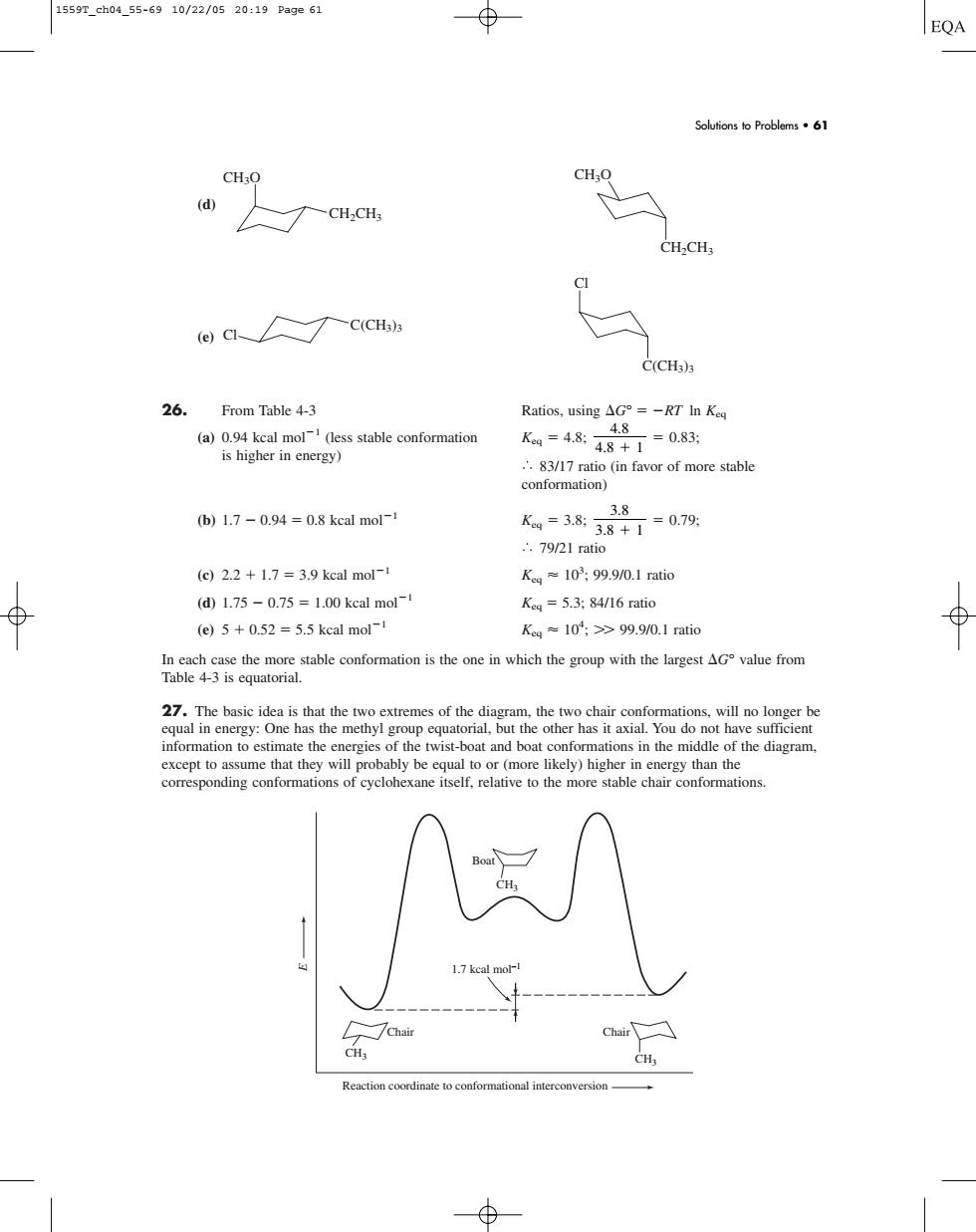
1559T_ch04_55-6910/22/0520:19Pa9e61 EQA Solfions to Problems61 d CH.CH ac、C(CH) C(CH3) 26.From Table 4-3 Ratios,using△G°=-RT In Keg K=484=083: ..837mi (b)1.7-0.94=0.8 kcal mol1 (g2.2+1.7=3.9 kcal mol Ka=10:99.9/0.1 ratic (d1.75-0.75=1.00 keal mol- Ke=5.3:84/16 ratio (e)5+0.52=5.5 kcal mol- Kea≈10;>99.90.1 ratio e e即油rot ahe information toestimate the of the twist-boat and boat conformations in the middle of the diagram except to assume that they will probably be equal to orresponding conformations of (d) (e) 26. From Table 4-3 Ratios, using G° RT ln Keq (a) 0.94 kcal mol1 (less stable conformation Keq 4.8; 0.83; is higher in energy) 83/17 ratio (in favor of more stable conformation) (b) 1.7 0.94 0.8 kcal mol1 Keq 3.8; 0.79; 79/21 ratio (c) 2.2 1.7 3.9 kcal mol1 Keq 103 ; 99.9/0.1 ratio (d) 1.75 0.75 1.00 kcal mol1 Keq 5.3; 84/16 ratio (e) 5 0.52 5.5 kcal mol1 Keq 104 ; 99.9/0.1 ratio In each case the more stable conformation is the one in which the group with the largest G° value from Table 4-3 is equatorial. 27. The basic idea is that the two extremes of the diagram, the two chair conformations, will no longer be equal in energy: One has the methyl group equatorial, but the other has it axial. You do not have sufficient information to estimate the energies of the twist-boat and boat conformations in the middle of the diagram, except to assume that they will probably be equal to or (more likely) higher in energy than the corresponding conformations of cyclohexane itself, relative to the more stable chair conformations. E Reaction coordinate to conformational interconversion CH3 CH3 CH3 Chair Chair Boat 1.7 kcal mol–1 3.8 3.8 1 4.8 4.8 1 Cl C(CH3)3 C(CH3)3 Cl CH3O CH2CH3 CH2CH3 CH3O Solutions to Problems • 61 1559T_ch04_55-69 10/22/05 20:19 Page 61��������������