正在加载图片...
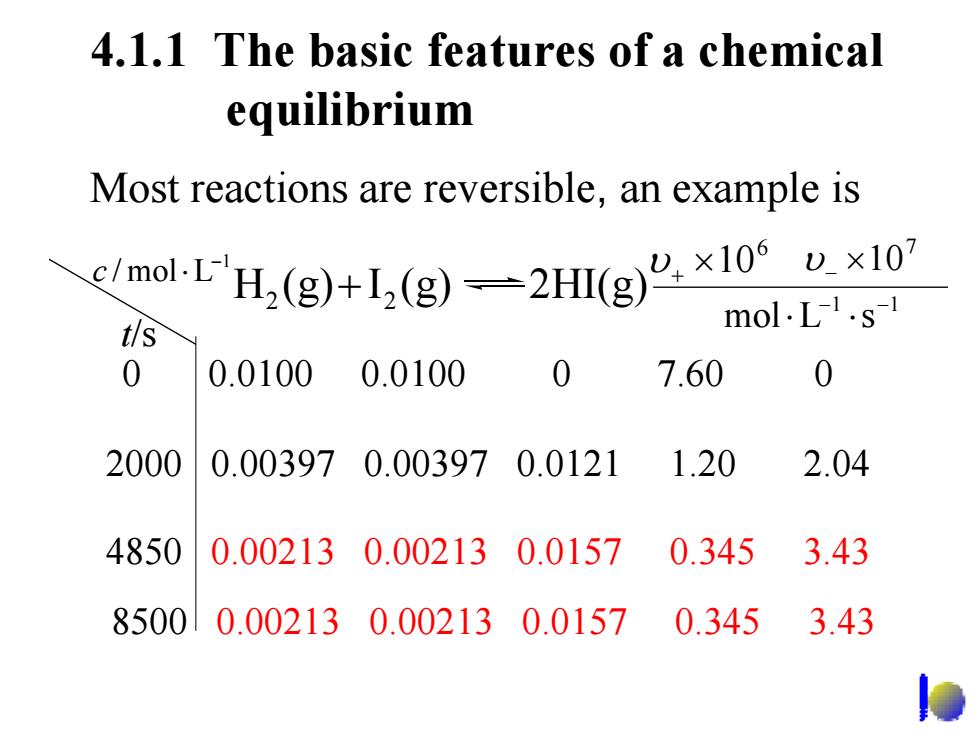
4.1.1 The basic features of a chemical equilibrium Most reactions are reversible,an example is emo1-EH,(g)+1,(g)=2Hg×10ux10 mol.L.s-1 t/s 0 0.0100 0.0100 0 7.60 0 2000 0.003970.003970.0121 1.20 2.04 4850 0.002130.002130.0157 0.3453.43 85000.002130.002130.01570.345 3.43 4.1.1 The basic features of a chemical equilibrium 0 0.0100 0.0100 0 7.60 0 2000 0.00397 0.00397 0.0121 1.20 2.04 4850 0.00213 0.00213 0.0157 0.345 3.43 Most reactions are reversible, an example is t/s 1 / mol L− c ⋅ 6 υ + ×10 7 υ − ×10 1 1 mol L s − − ⋅ ⋅ H (g) I (g) 2HI(g) 2 + 2 8500 0.00213 0.00213 0.0157 0.345 3.43